An Efficient One-Pot Synthesis of Dihydropyrimidinones Under Solvent-Free Conditions
Farhad Hatamjafari , Fatemeh Germani Nezhad*
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran
DOI : http://dx.doi.org/10.13005/ojc/300148
Article Received on :
Article Accepted on :
Article Published : 03 Mar 2014
An efficient method for one-pot synthesis of 3,4-Dihydropyrimidin-2-(1H) -one derivatives the Biginelli condensation reaction of aromaticaldehydes, β-ketoesters and urea under solvent-free conditions was described.
KEYWORDS:Biginelli Reaction; Dihydropyrimidinones; One-pot; Solvent-Free
Download this article as:| Copy the following to cite this article: Hatamjafari F, Nezhad F. G. An Efficient One-Pot Synthesis of Dihydropyrimidinones Under Solvent-Free Conditions. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Hatamjafari F, Nezhad F G. An Efficient One-Pot Synthesis of Dihydropyrimidinones Under Solvent-Free Conditions. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2260 |
Introduction
Multicomponent reactions play an important role in pharmaceutical industries. Pharmacies are trying to develop green chemistry reactions. Solvent-free synthesis of complex organic structures as drugs is the dream of every pharmacy. Multicomponents reaction as a powerful tool for the rapid introduction of molecular diversity is evident and developed for the generation of heterocycles receives growing interest1-3.
Dihydropyrimidinones (DHPMs) as well as heterocyclic compound in the natural and synthetic organic chemistry due to their wide range of biological and therapeutic properties such as anti-viral anti-inflammatory, anti-tumor, and anti-bacterial activities4-5. Biginelli reaction is one of the most important multi-component reactions for the synthesis of dihydropyrimidinones. 3,4-dihydropyrimidin-2-(1H) ones (DHPMs) reported that the activity of many drugs as anti-viral, anti-bacterial and anti-hypertensive effects as calcium channel modulators and as Multi-drug resistance reversal. Biological activity of some alkaloids isolated recently to 3,4 dihydropyrimidin-2-(1H) -ones moiety. It was first synthesized by Biginelli in 1893. DHPMs as a pot condensation of an aldehyde, diketone and urea under acidic conditions. This method undergoes from low yields especially in the cases of some substituted aldehydes. To increase the efficiency of the reaction, Biginelli, various catalysts have been used6-8. However, these reactions often require harsh conditions and long reaction time and low efficiency can. Aliphatic and aromatic aldehydes, especially when used to replace for synthesis Dihydropyrimidinones (DHPMs). Although numerous methods are capable of affecting these synthesis has been previously reported9-18. Previously, we have synthesized a number of heterocyclic compounds19-30. Herein we report some of DHPMs at one pot reaction, environmentally friendly with high yields and easy separation (Scheme 1)
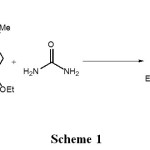 |
Schem 1: Click here to View Schem |
Experimental
General Procedure for the Preparation of ethyl 1,2,3,4-tetrahydro-6-methyl-2-oxo-4-phenylpyrimidine-5-carboxylate
A mixture of ethyl acetoacetate (1 mmol), benzaldehyde (1 mmol) and urea (1.4 mmol) was heateded for 90 min. All reactions were monitored by TLC and the obtained solid was filtered, the solid was washed with water and recrystallized using absolute ethanol.

RESULTS AND DISCUSSION
Herein, we reported an efficient, high yield, environmentally friendly, easy separation and simple route for the synthesis of DHPMs under solvent-free conditions.
ACKNOWLEDGEMENTS
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
References:
- Ma N., Jiang B., Zhang G., Tu S. J., Wever W and Li G., Green Chemistry, 12: 1357 (2010).
- Yue T., Wang M. X., Wang D.X., Masson G and Zhu J., J. O. Chem., 74: 8396 (2009).
- Trofimov B. A., Andriyankova L. V., Belyaeva K. V et al., Eur. J. O. Chem., 9: 1772 (2010).
- Balme G., Bossharth E and Monteiro N., Eur. J. Org.Chem., 21: 4101 (2003).
- Kappe, C.O. Tetrahedron, 49: 6937 (1993).
- Kappe, C.O. Acc. Chem. Res., 33: 879 (2000).
- Snider, B.B.; Chen, J.; Patil, A.D.; Freyer, A. Tetrahedron Lett., 37: 6977 (1996).
- Biginelli, P. Gazz. Chim. Ital., 23: 360 (1893).
- Banik, B.K.; Reddy, A.T.; Datta A.; Mukhopadhyay C. Tetrahedron Lett., 48: 7392 (2007).
- Li, J.T.; Han, J.F.; Yang, J.H.; Li, T.S. Ultrason. Sonochem., 10: 119 (2003).
- Peng, J.J.; Deng, Y.Q. Tetrahedron Lett., 42: 5917 (2001).
- Ma, Y.; Qian, C.; Wang, L.; Yang, M. J. Org. Chem., 65: 3864 (2000).
- Ahmed, N.; Lier, J.E.V. Tetrahedron Lett., 48: 5407 (2007).
- Adib, M.; Ghanbary, K.; Mostofi, M.; Ganjali, M.R. Molecules, 11: 649 (2006).
- Karade, H.N.; Sathe, M.; Kaushik, M.P. Molecules, 12:1341 (2007).
- Cheng, J.; Qi, D.Y. Chin. Chem. Lett., 18: 647 (2007).
- Liu, C.J.; Wang, J.D.; Li, Y.P. J. Mol. Catal. A Chem., 258: 367 (2006).
- Zhang, X.L.; Li, Y.P.; Liu, C.J.; Wang, J.D. J. Mol. Catal. A Chem., 253: 207 (2006).
- Azizian J., Hatamjafari F., Karimi A. R. and Shaabanzadeh M., Synthesis, 5: 765 (2006).
- Azizian J., Shaabanzadeh M., Hatamjafari F. and Mohammadizadeh M.R., Arkivoc, (xi): 47 (2006).
- Hatamjafari F., Synthetic Communications, 36: 3563 (2006).
- Azizian J., Hatamjafari F. and Karimi A. R., Journal of Heterocyclic Chemistry, 43: 1349 (2006).
- Hatamjafari F and Montazeri N., Turkish Journal of Chemistry, 33: 797 (2009).
- Bidram A., Hatamjafari F and Doryeh A., Orient. J. Chem., 29: 123 (2013).
- Hatamjafari F., Orient. J. Chem., 28: 141 (2012).
- Hatamjafari F., Orient. J. Chem., 29: 93(2013).
- Hatamjafari F and Alijanichakoli F., Orient. J.Chem., 29: 145(2013).
- Hatamjafari F and Hosseinian A., Orient. J.Chem., 29: 109(2013).
- Hatamjafari F and Keyhani A., Orient. J.Chem., 29: 783 (2013).
- Hatamjafari F and Khojastehkouhi H., Orient. J. Chem., 30: (2014 in press).

This work is licensed under a Creative Commons Attribution 4.0 International License.









