Ultrasound-assisted the three-component synthesis of spiro[4H-pyrano[3,2-c]quinolin-4,3′-indoline]-2′,5(6H)-diones in water
Shaghyegh Gholizadeh and Koorosh Radmoghadam*
Chemistry Department, Islamic Azad University, Tonekabon branch, Tonekabon, Iran
DOI : http://dx.doi.org/10.13005/ojc/290450
Article Received on :
Article Accepted on :
Article Published : 28 Dec 2013
A simple and efficient synthesis of the titled spirooxindoles was accomplished via the three-component reaction between isatins, 4-hydroxy-2H-quinolin-2-one, and malononitrile or ethylcyanoacetate in aqueous medium under ultrasound irradiation.
KEYWORDS:Spirooxindole;Three-component reaction; Ultrasound irradiation; In water reaction
Download this article as:| Copy the following to cite this article: Gholizadeh S, Radmoghadam K. Ultrasound-assisted the three-component synthesis of spiro[4H-pyrano[3,2-c]quinolin-4,3′-indoline]-2′,5(6H)-diones in water. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Gholizadeh S, Radmoghadam K. Ultrasound-assisted the three-component synthesis of spiro[4H-pyrano[3,2-c]quinolin-4,3′-indoline]-2′,5(6H)-diones in water. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1304 |
INTRODUCTION
Water, after its unique role as solvent in nature, is gaining more and more importance in the recent researches of environmentally benign chemical reactions. It is a cheap and inherently safe solvent relative to more traditional organic liquids. Hydrophobic interactions of water with organic substrates were shown to be the origin of selectivity and rate enhancements of organic reactions in watery solutions or even suspensions.1 These fascinating features delineate a wide perspective for application of water as solvent in environmentally benign organic syntheses, especially when matched with a clean method of activation such as ultrasound irradiation. Ultrasonic irradiation is a safe transfer of activation energy to reacting molecules providing a unique distribution profile of penetrating waves in matter giving rise to acceleration of numerous catalytic reactions in homogeneous and heterogeneous systems.2-3 The indole moiety is probably the most known heterocycle, a common and important feature of a variety of natural products and medicinal agents.4 Among the indole-based heterocycles, those compounds consisted of spirooxindole core represent an important class of naturally occurring substances characterized by highly pronounced biological properties.5 On the other hand, pyranoquinoline derivatives are found to possess a wide spectrum of biological activities, such as psychotropic, antiallergenic, anti-inflammatory, and estrogenic activity.6 Furthermore, several pyranoquinoline containing alkaloids exhibit cancer cell growth inhibitory activity and are investigated as potent anticancer agents.7-8 In this background and in line with ongoing researches devoted to synthesis of spirooxindole compounds,9 we report an efficient one-pot method for synthesis of spiro[4H-pyrano[3,2-c]quinolin-4,3′-indoline]-2′,5(6H)-diones in aqueous media under ultrasound irradiation.
EXPERIMENTAL
Typical procedure for the preparation of (4a):
A tube was charged with 4-hydroxy-2H-quinolone (0.16 g, 1 mmol), isatin (0.15 g, 1 mmol), malanonitrile (0.06 g, 1 mmol), piperidine (5 mol %) and water (5 mL). The reaction mixture was sonicated at 50 ºC for 5 min. After completion of reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, filtered and the solids were wash with water and ethanol to afford the product 4a as white powder (86 %). A library of products (4a-j , see Table 3) were efficiently synthesized by this method among them 4a-e and 4i-j are known compounds,10 but the products 4f-g are reported here for the first time.
Selected data for Ethyl 2-amino-5′-methoxy-2′,5-dioxo-5,6-dihydro-spiro[pyrano[3,2-c]quinoline-4,3′-indoline]-3-carboxylate (4f): Cream powder, Yield, 0.40 g (92 %). mp> 300 oC, IR (KBr), νmax: 3442, 3340, 3180, 2940, 2860, 1687, 1654, 1612, 1480, 1348, 1298, 1104 cm-1. 1H NMR (400.13 MHz, DMSO-d6) δ: 0.87 (t, 3H, J 7.2 Hz, OCH2CH3), 3.59 (s, 3H, O-CH3), 3.73-3.81 (m, 2H, OCH2CH3), 6.54 (d, 1H, J 2.4 Hz, 4′-H), 6.60 (d, 1H, J 8.4 Hz, 7′-H), 6.65 (dd, 1H, J 8.4 and 2.4 Hz, 6′-H), 7.30 (t, 1H, J 7.6 Hz, 9-H), 7.31 (d, 1H, J 7.6 Hz, 7-H), 7.59 (t, 1H, J 7.6 Hz, 8-H), 8.02 (d, 1H, J 7.6 Hz, 10-H), 8.05 (s, 2H, NH2), 10.10 (s, 1H, NH), 11.51 (s, 1H, NH). 13C NMR (100.61 MHz, DMSO-d6) δ: 13.6 (OCH2CH3), 48.6 (Cspiro), 55.7 (OCH3), 59.4 (OCH2CH3), 76.4 (C-C≡N), 108.6, 109.9, 110.4, 112.0, 112.2, 115.4, 122.3, 122.6, 132.0, 137.3, 138.1, 138.6, 151.7 (C-5′), 154.8 (C-4b), 159.6, 159.8 (C-2 and C-5), 168.1 (C=O ester), 179.9 (C=O oxindoline). MS (EI) m/z (%): 433 (M+, 1), 418 (M+-CH3, 4), 405 (M+-CH2=CH2, 5), 390 (2), 374 (100), 346 (25), 331 (14), 299 (8), 272 (15). Anal. Calcd for C23H19N3O6: C, 63.74; H, 4.42; N, 9.70%.Found: C, 63.81; H, 4.49; N, 9.63%.
Selected data for 2-Amino-5′-methoxy-6-methyl-2′,5-dioxo-5,6-dihydro-spiro[pyrano[3,2-c]quinoline-4,3′-indoline]-3-carbonitrile (4g): Cream powder, Yield, 0.35 g (88%). mp> 300 oC, IR (KBr), νmax: 3530, 3400, 3300, 3155, 2198, 1709, 1672, 1621, 1590, 1485, 1360 cm-1. 1H NMR (400.13 MHz, DMSO-d6) δ: 3.48 (s, 3H, N-CH3), 3.63 (s, 3H, OCH3), 6.68 (br s, 1H, 4′-H), 6.74-6.75 (m, 2H, 6′-H and 7′-H), 7.43 (t, 1H, J 7.6 Hz, 9-H), 7.46 (s, 2H, NH2), 7.58 (d, 1H, J 8.4 Hz, 7-H), 7.75 (dt, 1H, J 7.8 and 1.6 Hz), 8.06 (dd, 1H, J 8.0 and 1.6 Hz, 10-H), 10.36 (s, 1H, NH). 13C NMR (100.61 MHz, DMSO-d6) δ: 29.7, 49.1 (Cspiro), 55.8 (OCH3), 57.8 (C-C≡N), 106.9, 110.0, 110.8, 112.8, 113.4, 115.5, 118.0, 122.8, 122.9, 132.7, 136.0, 136.3, 139.2, 152.0 (C-5′), 155.5 (C-4b), 159.2, 159.3 (C-2 and C-5), 178.2 (C=O oxindoline). MS (EI) m/z (%): 400 (M+, 9), 381 (2), 368 (1), 322 (4), 236 (9), 216 (15), 149 (17), 97 (79), 57 (100). Anal. Calcd. for C21H14N4O4: C, 66.00; H, 4.03; N, 13.99 %. Found: C, 65.91; H, 4.09; 14.03 %.
Selected data for 2-Amino-6-methyl-5′-nitro-2′,5-dioxo-5,6-dihydro-spiro[pyrano[3,2-c]quinoline-4,3′-indoline]-3-carbonitrile (4h): Creamy powder, Yield, 0.33 g (80%). mp> 300 oC, IR (KBr), νmax: 3445, 3304, 3250, 3196, 2198, 1736 (C=O), 1674 (C=O), 1622, 1583, 1506, 1364, 1337, 1160 cm-1. 1H NMR (400.13 MHz, DMSO-d6) δ: 7.06 (d, 1H, J 8.8 Hz, 7′-H), 7.47 (t, 1H, J 7.4 Hz, 9-H), 7.61 (d, 1H, J 8.4 Hz, 7-H), 7.68 (s, 2H, NH2), 7.78 (dt, 1H, J 7.8 and 1.2 Hz, 8-H), 8.08 (dd, 1H, J 8.0 and 1.2 Hz, 10-H), 8.11 (d, 1H, J 2.4 Hz, 4′-H), 8.18 (dd, 1H, J 8.4 and 2.4 Hz, 6′-H), 11.31 (s, 1H, NH). 13C NMR (100.61 MHz, DMSO-d6) δ: 29.8 (CH3), 48.8 (Cspiro), 56.1 (C-C≡N), 105.7, 109.8, 112.9, 115.6, 117.7, 120.0, 123.02, 123.05, 126.3, 132.9, 135.8, 139.2, 143.0 (C-5′), 149.5 (C-7’a), 152.6 (C-4b), 159.4 (C-2), 159.7 (C=O), 179.0 (C=O oxindoline). MS (EI) m/z (%): 415 (M+, 0.5), 381 (9), 368 (12), 339 (8), 330 (8), 313 (28), 264 (20), 236 (30), 194 (100), 175 (33). Anal. Calcd for C21H13N5O5: C, 60.72; H, 3.15; N, 16.86 %. Found: C, 60.76; H, 3.22; N, 16.73 %.
RESULTS AND DISCUSSION
At the onset of this study, the reaction of isatin, 4-hydroxy-2(1H)-quinolone and malononitrile was used as the model to explore the optimum reaction conditions (Table 1).
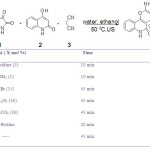 |
Table 1. Optimization of reaction conditions for the model reaction in 1:5 ethanol:water solution under ultrasound irradiation (45 KHz). Click here to View table |
As Table 1 show, better results were obtained with the aid of basic catalysts especially piperidine and triethylamine. In the absence of any catalyst, the reaction takes a longer time and gives lower yield. Upon further examination of the trial reaction, water has been proved to be the superior solvent in spite of its lower solvability (see Table 2). The best result was obtained with piperidine (5 mol %) as catalyst under ultrasonic irradiation for 5 min at 50 ºC. To verify the role of ultrasounds, the reaction was also run in water at 50 ºC without soniaction. As this table show, ultrasound irradiation improves the yield of the reaction (entry 2).
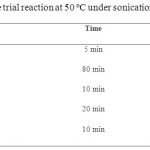 |
Table 2. Further optimizations of the trial reaction at 50 ºC under sonication (unless specified). Click here to View table |
Encouraged by this success, a variety of isatin derivatives, quinolones, and malononitrile or ethyl cyanoacetate were employed under similar circumstances to evaluate the applicability of this method. The results summarized in Table 3 show the viability of this method. It is evident from this table that both electron-deficient and electron-rich isatins afford fairly high yields of the desired spiro-condensates in reaction with malononitrile and a variety of 1,3-dicarbonyl compounds in a few minutes at ambient temperature. Nevertheless, the electron-donating methoxy group at the 5-position of isatin substrate renders the reaction to give slightly higher yields, whilst presence of the electron-accepting nitro group at the same position have a retardation effect on the rate of the reaction. Moreover, employing ethyl cyanoacetate in place of malononitrile furnishes similar connective assembly of spiro-annulated pyran-oxindole products. The general aspect of the present method partially lies on the fact that amidic N-H group of 4-hydroxy-2H-quinolone did not affect on the course of reactions, as N-substituted and unsubstituted quinolones take part similarly into the reaction to give the corresponding products. The structures of some products were confirmed by comparison of their IR and 1H NMR spectroscopic as well as physical data with those of authentic samples prepared from previously reported method,8 however the new products 4f-h were fully characterized by IR, 1H NMR, 13C NMR and Mass spectral data as well as their elemental microanalyses.
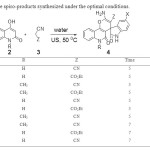 |
Table 3. The library of the spiro-products synthesized under the optimal conditions. Click here to View table |
The synthesis hinged on a cascade of reactions in which first isatin 1 condenses with malononitrile 3 to afford the intermediate isatylidenemalononitrile 5. This intermediate undergoes a Michael type addition at its exocyclic C=C bond with the 4-hydroxyquinolin-2(1H)-one 2 followed by a cyclization through addition of the OH and cyano groups existing on the resultant adduct (Scheme 1).
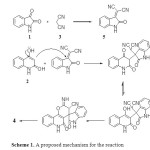 |
Scheme 1. A proposed mechanism for the reaction Click here to View table |
CONCLUSION
In conclusion, we have developed a quick, clean, and simple method for the synthesis of spiro[4H-pyrano[3,2-c]quinoline-4,3′-indoline]-2′,5(6H)-dione in water under ultrasound irradiation. Prominent among the advantages of this method are operational simplicity, good yields, easy workup and using water as solvent. The reactions gain acceleration and give better yields under ultrasound irradiation.
ACKNOWLEDGEMENTS
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
REFERENCES
- Chanda A., and Fokin V. V., Chem. Rev. 109: 725 (2009).
- (a) Mason T. J., and Peters D., Practical sonochemistry, second ed., Ellis Harwood, London, 2002. (b) Mashayekhi H.-A., Rezaee M., and Montazeri N., Orient. J. Chem. 28: 263 (2012).
- Hoalihan W. J., Remers W. A., Brown R. K., Indoles: Part 1; Wiley: New Yourk, NY, 1992.
- (a) Ma J., Hecht S. M., Chem. Commun. 1190 (2004). (b) Edmondson S., Danishefsky S. J., Sepp-Lorenzinol L., and Rosen N., J. Am. Chem. Soc. 121: 2147 (1999).
- (a) Abd E., Hisham A., Pharmazie 52: 28 (1997). (b) Chen I. S., Wu S. J., Tsai I. J., Wu T. S., Pezzuto J. M., Lu M. C., Chai H., Suh N., Teng C. M., J. Nat. Prod. 57: 1206 (1994).
- Chen T. S., Wu S. J., Tsai I. L., Wu T. S., Pezzuto J. M., Lu M. C., Chai H., Suh N., and Teng C. M., J. Nat. Prod. 57: 1206 (1994).
- Rad-Moghadam K., and Youseftabar-Miri L., Synlett 11: 1969 (2010).
- Rad-Moghadam K., and Youseftabar-Miri L., Tetrahedron 67: 5693 (2011).

This work is licensed under a Creative Commons Attribution 4.0 International License.









