Synthesis of Indole Annulated Benzazepine Derivative of Medicinal Interest
Aditi Vandana and D.Kishore
Department of Chemistry,Banasthali University,Banasthali-304022(India)
DOI : http://dx.doi.org/10.13005/ojc/290451
Article Received on :
Article Accepted on :
Article Published : 08 Jan 2014
The ubiquity of benzazepines in medicinal chemistry is undoubtedly a consequence of the multifarious biological response which they elicit in combating a variety of body ailments.A benzazepine derivative-‘Fenoldopam mesylate’has emerged as avasodilator in peripheral arteries ansd as a diuretic in the kidneys. This discovery provided an optimism for the search of other novel agents from benzazepine class of compounds which could show a higher level of medicinal efficacy.The proposed synthesis (scheme-1) , in its first step was propelled forward with the formation of compound 1.3(which resulted from the reaction of succinyl chloride and p-fluoroaniline).The reaction of 1.3 with ethyl formate in the presence of a base formed 1.4 which underwent Japp-Klingemann reaction with aryl diazonium chloride followed by cyclocondensation with acid under the conditions of Fischer indolization to give indole substituted derivative 1.5.
KEYWORDS:P-fluoroaniline;Sunninyl chloride;Japp-Klingemann reaction;Fischer Indolization;Spectral study
Download this article as:| Copy the following to cite this article: Vandana A, Kishore D. Synthesis of Indole Annulated Benzazepine Derivative of Medicinal Interest. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Vandana A, Kishore D. Synthesis of Indole Annulated Benzazepine Derivative of Medicinal Interest. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1323 |
Introduction
Benzazepine moiety occurs frequently in both natural and synthetic drugs and is of highly biological interest. The use of this class of compound is not merely confined to the management of stress related conditions and for their use as antibacterial agents (1,3),but their additional applications are continuously emerging.Galantamine(Galanthamine), a benzazepine derived from norbelladine,has been studied in the treatment for Alzheimer disease(4).In a quest to develop easily accessible routes to the synthesis of some novel heteroring annulated benzazepine derivatives, the present investigation aims to examine the operational feasibility of the proposed strategy outlined in scheme-1 for thesynthesis of compounds I.6-1.7.
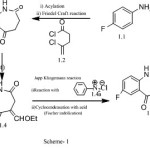 |
Scheme 1 Click here to View Scheme |
Materials and method:
P-fluoroaniline and succinyl chloride were the product of Fischer Scientific.All the chemicals employed for synthesis were of analytical grade and were used as supplied without further purification.All the solvents were dried and distilled before use. Melting points were determined in open glass capillaries and are uncorrected.The purity of the compounds was checked by TLC on silica gel(G) plates .The visualization of spot was carried in an iodine chamber..IR spectra were recorded on CE(SHIMADZU)FTIR-8400S.1 H NMR spectra were recorded on model AC-300F(Brucker) using CDCl3/DMSO-d6 as solvent and TMS as an internal reference.Chemical shift are expressed in δppm.
Synthesis of (7-fluoro-3,4-dihydro-1H-benzo[b]azepine-2,5-dione) (compound-1.3):
Acylation:
Compound 1.1 (2.20g)was mixed with succinyl chloride 1.2(1.64g) and dry pyridine(5ml).The mixture was refluxed for 10-15 mins.Cooled reaction mixture was poured slowly with stirring in 150-200ml ice cold water.The resulting solid reactant which was settled was filtered , washed with cold water , recrysstalized from hot water containing few drops of methanol.
Friedel craft reaction:
Compound above prepared(2.58 g)was mixed with PPA(5g) and heated at 150-160oC for 4hr (the process of the reaction was monitored by TLC).The reaction mixture was cooled to 20oCand concenterated salt of Na2CO3 was added to make it alkaline.The product was extracted with ethyl acetate (3×10 ml).The extract was dried overNa2SO4 and concenterated in vaccum.The residue was purified by column chromatography of silica gel with CHCl3 as an eluent to give compound 1.3.yield60%,m.p.120-122 oC.
Synthesis of 4-(ethoxymethylene)-7-fluoro-3,4-dihydro-1H-benzo[b]azepine-2,5-dione
(compound-1.4):
To a solution of 10% sodium ethoxide(10 ml) in benzene (50ml) at 0oC,a solution of ethyl formate (10 mml) in dry benzene (25 ml) was added. To this mixture compound 1.3 (2.25g) in benzene (25ml)was added.The mixture was stirred for4 hrs at room temperature and allowed to stand overnight.It was then diluted with cold water, acidified with dil.HCl and extracted with ether.The solvent was evaporated and the resultant compound (1.4) was recrystallised from ethanol to give pure compound 1.4.yield 50%, m.p.298-300oC.
Synthesis of 8-fluorobenzo[6,7]azepino[4,3-b]indole-6,12(5H,1H)-dione (compound-1.5):
Preparation of hydrazone:
A solution of Compound1.4a (3.26) was kept aside for 10 minutes.It was then added portionwise to an ice cold mixture containing compound 1.4 (4.0ml) , sodium acetate(6g), methanol(37.0ml) and water(20.0ml) over a period of 0.5 hours with stirring.The contents were allowed to stand for further 0.5 hours and the resulting solid mass was filtered , washed with water, dried and crystallized from ethanol.
Cyclization of hydrazone:
A solution of hydrazone above prepared (3.18g) in a mixture of acetic acid(19.0ml) and hydrochloric acid(4.7 ml)was refluxed on an oil-bath preheated to 125-130oC for 0.5 hours.The contents were then cooled and poured into cold water with stirring.The separated brown solidwas purified by passing through a column of silica gel using 50% benzene in petroleum ether as eluent.yield 66%,m.p.133-135 oC.
Results and discussion:
Scheme – 1 envisaged that the reaction of 1.3 with ethyl formate in presence of a base formed 1.4 which undergo Japp-Klingemann reaction with aryl diazonium chloride followed by cyclocondensation with acid under the conditions of Fischer indolization to give indole ring substituted derivative 1.5
IR spectra
Infrared spectra of compound 1.3 on KBr pellet exhibited strong absorption bands at 1660cm– and 1700cm– for the carbonyl group of CONH and CO groups respectively,along with this IR spectum also exhibited bands at 3400cm– (NH),[2970,1430cm– (CH2 next to C=O)],2970cm– (C-H),1590cm– (C=C)..
Infrared spectra of compound 1.4 on KBr pellet exhibited peaks at 3068cm– (C-H str. of C6H5F) ,1550cm– (C=C str. of C6H5F),3280cm– (NH str.) [CONH],1680cm– (C=O)[CONH],1610cm– (C=O str.),1620cm– (C=C).
Infrared spectra of compound 1.5 on KBr pellet exhibited peaks3068cm– (C-H str. of C6H5F) , 1550cm– (C=C str. of C6H5F),3280cm– (NH str.) [CONH],1680cm– (C=O)[CONH],1610cm– (C=O str.),3300cm– (NH sec.amine).The formation of compound 1.5 was confirmed by appearance of peak at 3300 cm– ( NH of indole ring) .
H NMR spectra
1H NMR spectrum of compound 1.3 in CDCl3+DMSO-d6 displayed signals for the presence of 8 protons.Appearence a downfield broad singlet for 1H at δ8.0 was attributed to NH group.Appearence of multiplet at δ 7.40-7.87wasassigned to the three protons of fluorobenzene ring.A singlet for four protons which appeared at δ2.93 and δ2.42 was attributed to two CH2 groups of azepanedione ring.
1H NMR spectrum of 1.4in CDCl3+DMSO-d6 exhibited a downfield broad singlet for 1H at δ8.0 which was attributed to NH group of azepanedione ring.Another singlet for 1H at δ 6.95 was assigned to the protons of CH group attached to ether group on one side and α ,β–unsaturated carbonyl group on other side.Presence of a triplet for 3H at δ1.21 and quartet for 2H at δ4.49 were assigned to the ethyl group of enolic ether. .Appearence of multiplet at δ7.45-7.98 wasassigned to the three protons of fluorobenzene ring. A singletfor two protonswhich appeared at δ2.90 was attributed to CH2 group of azepanedione ring.
1H NMR spectrum of 1.5in CDCl3+DMSO-d6 exhibited a downfield broad singlet for 1H at δ8.0 which was attributed to NH group of azepanedione ring..Appearence of multiplet at δ 7.45-7.98 wasassigned to the three protons of fluorobenzene ring. .Another singlet for 1H at δ 11.63 was assigned to NH group of indole. Appearence of multiplet at δ 6.91-8.47wasassigned to the four protons of benzene ring .
Mass spectra
Mass spectrum of compound 1.3 gave peaks at m/z193.05(100.0%),194.06(11.0%)which provided a strong evidence to its molecular weight(193.17).
Mass spectrum of compound 1.4 gave peaks at m/z 249.08(100.0%),250.08(14.5%)which provided a strong evidence to its molecular weight(249.24).
Mass spectrum of compound 1.5 gave peaks at m/z 310.32(100.0%),218.363(15.5%),239.99(11.1%),279.552(9.8%),291.112(5.4%) provided strong evidence to its molecular weight(310.32).
Acknowledgements
Authors are thankful to the university authorities for providing laboratory facilities.
References
- Johnson P.D., Aristoff P.A., Zurenko G.E., Schaadt R.D.,Yagi B.H.,Ford C.W., Hamel J.C., Stapert D., Moerman J.K., Synthesis and Biological Evaluation of Benzazepine Oxazolidinone Antibacterials, Bioorg. Med. Chem. Lett ., 2003;13:4197-4200.
- Bal Krishan, Om Prakash and E. H. El-Mossalamy., Orient. J. Chem., 29(1), 381-388 (2013)
- Das J., Sitaram Kumar M., Subrahmanyam D.,Sastry T.V.,Prasad Narasimhulu C.,Laxman Rao C.V., Kannan M., Roshaiah M.,Awasthi R.,Patil S..N.,Sarnaik H.M., Rao Mamidi,Selvakumar N.V.,Iqbal J., Substituent Activity Relationship Studies on New Azolo Benzoxazepinyl Oxazolidinones, Bioorg. Med. Chem .,Lett.,2006; 14 (23): 8032-42.
- Olin J.,Schneider L., Galantamine for Alzheimer’s Disease, Cochrane Database Syst Rev. CD001747 ,2002; 3.

This work is licensed under a Creative Commons Attribution 4.0 International License.









