Synthesis and Characterization of Divalent Transition Metal Complexes Containing Thiosemicarbazone Ligands
(Dr.) Prem Mohan Mishra
Professor in Chemistry , (MLSMCollege) L.N.MithilaUniversity, Darbhanga Bihar – 846004
DOI : http://dx.doi.org/10.13005/ojc/290453
Article Received on :
Article Accepted on :
Article Published : 16 Jan 2014
Complexes of divalent transition metals Co(II), Ni (II) and Cu(II) with ligand 2 – hydroxy – 4 – nitro acetophenone thiosemicarbazone have been synthesized. The complexes were characterized on the basis of elemental analysis, magnetic studies, electrical conductance and IR and electronic spectra. The complexes of Co(II) and Ni(II) were found to be octahedral where as Cu(II) complexes has square planer geometry.
KEYWORDS:Thiosemicarbazone; Schiff’s bases; Transition metals; complex compounds
Download this article as:| Copy the following to cite this article: Mishra P. M. Synthesis and Characterization of Divalent Transition Metal Complexes Containing Thiosemicarbazone Ligands. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Mishra P. M. Synthesis and Characterization of Divalent Transition Metal Complexes Containing Thiosemicarbazone Ligands. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1759 |
INTRODUCTION
Thiosemicarbazones posses considerable biological properties and have medicinal applications such as anti T.B. and leprosy1-2, antiviral properties3, antitumour4, anticancer5,6. Literature survey reveals that the presence of a metal ion increases the activity of or mitigate the side effects of the parent organic compounds7-10.
In continuation of our previous work in this communication we are going to report synthesis and characterization of complexes of Co(II), Ni(II) and Cu(II) with ligands containing thio semicarbozone of substituted aromatic ketones.
EXPERIMENTAL
Preparation of Ligand
4.8 g of 2 – hydroxy – 4 nitro – acetophenone was dissolved in methanol and treated with a solution containing 3.8 g BDH Anal R grade thio semicarbazide hydrochloride in water.
The mixture was stirred well and refluxed for half an hour on a water bath and cooled. Pale yellow crystals was separated. The crude yellow product was recrystallised from alcohol as colourless needles. Meeting point of the compound
was determined by Kjeldahl’s method and was found to be 491 K. The pure and dried compound was chemical by analysed. It is slightly soluble in alcohol but highly soluble in DMF but insoluble in water.
PREPARATION OF COMPLEXES.
Calculated quantity of metal salts (0.482 g cobalt chloride CoCl2.6H2O) was dissolved in methanol while the ligand (HNAPTS) was dissolved in DMF. Both the solution were mixed together and 1.0 g sodium acetate was added to it. The solution was refluxed for about three hours and left overnight. The grey precipitate was obtained. It is filtered and washed with methanol and ice cold water. The pure complexes was dried in air.
0.520g of nickel (II) chloride was dissolved in water while 0.820 g ligand was dissolved in DMF. Both the solution were mixed. 1.0 g sodium acetate was added and refluxed for about three hours and left to stand for an hour. The red coloured precipitate was obtained. It was filtered and washed with water and ethanol and dried in air
An ammoniacal solution of 0.256 g copper (II) chloride was treated with a solution of 0.348 g ligand (HNAPTS) dissolved in DMF. The red coloured precipitate separated was digested on the water bath for two hours and then left to stand for an hour. It was filtered washed with water and dried in air.
All the three complexes were analysed chemically.
RESULTS
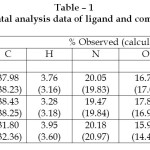 |
Table – 1: Elemental analysis data of ligand and complexes Click here to View table |
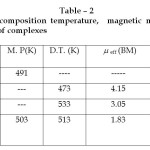 |
Table – 2 : Melting point, Decomposition temperature, magnetic moment & electrical conductivity data of complexes Click here to View table |
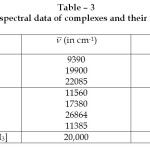 |
Table – 3: Electronic spectral data of complexes and their assignment Click here to View table |
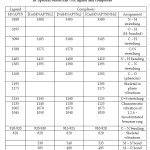 |
Table – 4: IR Spectral bands (cm-1) of ligand and complexes Click here to View table |
DISCUSSION
PROBABLE STRUCTURE OF LIGAND
2 – HYDROXY – 4 – NITRO ACETOPHENONE THIOSEMICARBAZONE, (HNAPTS)
The interpretation of I. R. spectra is quite complicated due to the presence of various similar groups and hence presence of many absorption bands. However, comparison of the spectral bands of the ligand (HNAPTS), with those of its complexes gives some important information regarding the nature of the lignad as well as the co-ordination sites through which metal ion has co-ordinated with the ligand.
The band at 3195 cm-1 in the ligand assignable to phenolic O – H (hydrogen bonded) stretching frequency11 disappears in the Ni(II), Co (II) complexes showing deprotonation of phenolic proton. The ligand also shows strong band at 1260 cm-1 which may be attributed to the phenolic C – O vibration. A shift of this band to higher frequency (~ 1300 cm-1) in the complexes indicate chelation of the ligand to metal ion through phenolic oxygen.
The sharp band at 1580 cm-1 in the free ligand due to of Schif’s base residue shifts to the lower frequency at (~1565 cm-1) in the complexes showing co-ordination through the nitrogen atom. The lowering may be very small in some cases. The ligand band at 1165 cm-1 may be assigned to group and shows downward shifting in metal complexes, indicating participation of this group in co-ordination.
The absorption band at 3380 cm-1 due to group in the free ligand remains unaltered in the complexes, indicating non-participation of this group in co-ordination.
A band present at 1385 cm-1 in the I. R. spectra of the ligand is assigned12 to group due to nitro group. This band remains unchanged in all the complexes, suggesting non – participation of nitro group in co-ordination.
Thus, we come to a conclusion that the ligand, 2 – Hydroxy – 4 – nitro acetophenone thiosemicarbazone (HNAPTS) behaves as a tridentate ligand for the metal ions, co-ordinating through (i) phenolic oxygen (C – O), (ii) nitrogen atom of (C = N), of azomethine group and (iii) ketonic sulphur of C = S, as enolic group. Similar kind of situation has been reported by S. K. Thampy.13
This ligand is monoprotic in all the cases except Ni(II) and Cu (II) at higher pH (pH = 9). It has been observed that in these cases a band present at 1165 cm-1 in the free ligand disappears, perhaps due to deprotonation of the C = S (enolic ) proton.
Many workers14-20 have prepared complexes with Schiff’s base derived from substituted salicylaldehyde and reported non-participation of the substituents in the co-ordination. However, stability of the complex is certainly influenced by the presence of the substituents. These results are in good agreement with our previous observation.17-23 The ligand may be assumed to be tautomer of the following two probable structures.
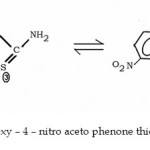 |
Fig. 1 : 2 – Hydroxy – 4 – nitro aceto phenone thiosemicarbazone Click here to View figure |
Structure of complexes
The molar conductance values of complexes 18.78 for Co(II), 16.80 for Ni(II) and 18.6 for Cu(II) suggest non–electrolytic nature of all the complexes [Co(HNAPTS)2], [Ni(HNAPTS)2] and [Cu(HNAPTS) NH3] .
Electronic Spectra: These Ni (II) complexes show four bands in their reflectance spectra favouring an octahedral stereochemistry.24 Out of these four bands, three can be assigned to spin allowed d – d transition, eg. 3A2g (F) ® 3T2g (F), 3A2g(F) ® 3T1g(F) and 3A2g(F) ®3T1g (P). The fourth band near 10750 cm-1 is probably spin forbidden25 and may be assigned to 2B1g ® 2Eg transition.
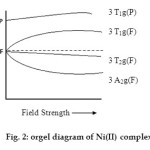 |
Fig. 2: orgel diagram of Ni(II) complex Click here to View figure |
Copper (II) has d9 configuration which makes Cu (II) subject to John – Teller distortion, if placed in an environment of cubic like regular octahedral or tetrahedral symmetry and this has a profound effect on all its stereochemistry26-27.
The typical distortion is an elongation along one four fold axis, so that there is planar array of four short Cu-L bonds with two trans long ones. In the limit of course, the elongation leads to a situation indistinguishable from square co – ordination as found in many discrete complexes of Cu (II) . Thus the cases of tetragonally distorted “Octahedral” co – ordination and square co – ordination cannot be sharply differentiated.
Because of the relatively low symmetry of the environments in which the Cu2+ ion is characteristically found, detailed interpretation of spectra and magnetic properties are somewhat complicated even though one is dealing with the equivalent of a one – electron case.
The molar conductance values suggest non – electrolytic nature of this complex while I.R. band near 655 cm-1 suggests M – NH3 bonding.
The magnetic moment of Cu (II) complex correspond well with the presence of one unpaired electron and gives on specific information about their stereochemistries. The eff value of Co(II) complex (4.15 BM) indicate the presence of three unpaired electron in the complex whereas eff for Ni(II) complex (3.05 BM) suggests presence of two unpaired electron in the complex.
Taking all these facts into consideration, along with their elemental analysis data the most probable structures of these complexes are given below in figure 3, 4 & 5.
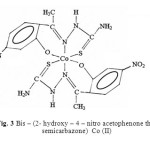 |
Fig. 3 Bis – (2- hydroxy – 4 – nitro acetophenone thio semicarbazone) Co (II) Click here to View figure |
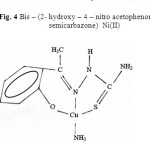 |
Fig. 4 Bis – (2- hydroxy – 4 – nitro acetophenone thio semicarbazone) Ni(II) Click here to View figure |
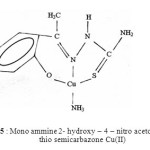 |
Fig. 5 : Mono ammine 2- hydroxy – 4 – nitro acetophenone thio semicarbazone Cu(II) Click here to View figure |
CONCLUSION:
Thiosemicarbazone metal complexes have been studied for a long time. The crystallographic data available to date is also very large but the study of the way thiosemicarbazone metal complexes interact with biological systems requires much attention. More light is needed on the grey zone between chemistry and molecular biology.
REFERENCES:
- Parekh,A K. and Desai K.K.: Indian J. Chem.2006; 45B; 1072.
- Chandra S. and Gupta L.K.: Spectrochimica Acta Part A.2005;62;1089.
- Demertzi D.K., Miller J.R., Kourkoumelis N., Hadzikaaou S.K. and Demertzis M.A: Polyhedron 1999;18;1005.
- Ferrari B.M., Fava G.G. Leporti E., Pelosi G., Rossi R., Tarasconi P., Albertini R., Bonati A., Lunghi P. and Pinelli S.: J. Inorganic Biochem.1998; 70;145.
- Ferrari M.B., Capacchi S., Reffo G., Aelosi G., Tarasconi P., Albertini R., Pinellis S., Lunghi P.: J. of Inorg. Biochem.2000; 81; 89.
- Bhat A.K., Bhamaria R.F., Patel M.R. Bellare R.A. and Deliwala C.V.: Indian J. chem.1972; 10; 694.
- Lobana T.S., Rekha, Butcher R.J., Castineiras A., Bermejo E. and Bharatam P.V.: Inorg. Chem.2006; 45; 1535.
- Sharma S., Athar F., Maurya M.R., Azam A.: European J. Med. Chem.2005; 40;1414.
- Klayman D.L., Bartosevich J.F., Griffine T.S., Mason C.J. and Scovill J.P: J. Med. Chem.1979; 22; 854.
- Klayman D.L., Scovill J.P., Bruce J. and Bartosevich J: J. Med. Chem.1984; 27; 84.
- Purushottam B.Chakrawarti and Pramila Khanna : J. Ind. Chem. Soc., 59, 828 (1982)
- A. Syamal and B.K.Gupta : J.Ind. Chem. Soc., 59, 697 (1982)
- S. K. Thampy, Ph.D. Thesis, BhavnagarUniversity, Gujrat
- B. K. Rai, Sapana Kumari, R. K. Singh, A. Prasad, M. P. Sinha & P. M. Mishra : Journal of Ultra Chemistry, Bhopal, M.P. Vol-5, No.- 1, p- 83-88, 2009
- Shiva Shakti, P.M. Mishra, S. K. Mishra, A. K. Jha: Asian Journal of Chemistry, Ghaziabad, U.P. Vol 22, No.- 7, 2010
- Shiva Shakti, Md. S. Raza, D. K. Jha & P. M. Mishra: Journal Of Ultra Chemistry, Bhopal, M.P. Vol. 7 No. 1, Page. 113-122, 2011
- P. M. Mishra, Shiv Shakti, S. K. Choudhary, Gunjan Vikash, G. Singh, J. P. Mishra & Sudha Kumari: Journal of Ultra Chemistry , Bhopal , M.P. Vol. – 8 , No. – 2, p- 195-204, August 2012
- P. M. Mishra, D. K. Choudhary, Shiva Shakti & J. J. Choudhary: Journal of Ultra Chemistry, Bhopal, M. P. Vol. 8 (3), 415 -420 (2012).
- P. M. Mishra : Journal of Ultra Chemistry, Bhopal (M.P), Vol. 8 (3), 401 -408 (2012).
- P. M. Mishra : Oriental Journal of Chemsitry, Bhopal, M.P. Vol. 29, No. 2, p- 677 – 683. 2013.
- Liberta A. E. and West D.X: BioMetal 1992; 5; 121.
- West D.X., Brain G.A., Jasinski J.P., Li Y., Pozdniakiv R.Y., Martinez J. V., Toscano R.A. and Ortega S. H.: Polyhedron 1996; 15; 665.
- A.B.P. Lever : “ Inorganic Electronic Spectroscopy”, Elsevier, London (1968)
- J.A. Bertrand and P.G. Eller : Inorg. Chem., (13) 927 (1974)
- S.A. Cotton : Co – ord. Chem. Rev., 8, 167 (1972)
- F. Cotton and G. Wilkinson : “ Advanced Inorganic Chemistry”, Weley Estern Ltd., London, VII Ed.(1984)
- B.J. Hathaway and D.E. Billing : Co – ordination Chem. Rev, 5, 143(1970)

This work is licensed under a Creative Commons Attribution 4.0 International License.









