Synthesis of Potassium 2,4 and 2,6-bis[(4,6-dimethoxypyrimidin-2-yl)oxy]benzoate
Samson Waghmare , Sanjeev Raut , Lokesh Gokhale and Pramod Kumar Sahu*
*R&D Dept., Godrej Agrovet Ltd., Pirojshanagar, Eastern Express Highway, Vikhroli (East) , Mumbai- 400079, India.
DOI : http://dx.doi.org/10.13005/ojc/290351
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The synthesis of Potassium 2,4 and 2,6-bis[(4,6-dimethoxypyrimidin-2-yl)oxy]benzoate , which belongs to the agrochemical field. The preparation method includes using 2-methylsufonyl-4, 6-dimethoxypyrimidine and 2,4- and 2,6-dihydroxy benzoic acid to directly react in aromatic hydrocarbon and in presence of potassium carbonate and potassium hydroxide to obtain above compounds . Both the compounds have herbicidal activity.
KEYWORDS:synthesis
Download this article as:| Copy the following to cite this article: Waghmare S, Raut S, Gokhale L, Sahu P. K. Synthesis of Potassium 2,4 and 2,6-bis[(4,6-dimethoxypyrimidin-2-yl)oxy]benzoate. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290351 |
| Copy the following to cite this URL: Waghmare S, Raut S, Gokhale L, Sahu P. K. Synthesis of Potassium 2,4 and 2,6-bis[(4,6-dimethoxypyrimidin-2-yl)oxy]benzoate. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=364 |
Introductions
Chemical weed control is a commonly used and reliable method to control weeds in direct seeded rice (DSR) and planted rice fields. Appropriate use of both pre-emergence and post emergence herbicides has been found effective in the paddy fields[1]. Since, DSR fields are characterized by floristically diverse weed communities[2]. The synthesis relates to a process for synthesis of a herbicidally active potassium salt of pyrimidinyloxy benzoic acid.
Chemical reaction
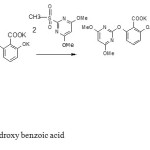
Scheme 1 |
Scheme 1 Click here to View figure |
Materials and Method
2,4 and 2,6-dihydroxy bezoic acid ( aldrich), 2-methylsulfony-4,6-dimethoxy pyrimidine ( Fulka) , potassium hydroxide ( Sdfine) , toluene (Sdfine) and benzyltriethyl ammonium chloride ( Aldrich) has been used in the reaction.
Toluene was charged in to 100ml RBF fitted with Dean& strack apparatus , mechanical stirrer and condenser Charged Dihydroxy Benzoic acid (1.0 gm-mole) followed PTC 5% and potassium hydroxide ( 3.0 gm-mole) at room temperature. Reflux for 2 hrs and cooled the reaction mass to 70 OC and then 2-Methylsulfonyl -4,6-dimethoxy pyrimidine( 2.0 gm-mole) was added. Reaction was maintained at Reflux temperature for 10 hrs. Product was isolated by filtration and recrystalized form ethyl acetate. Purity of the product >99.0% having yield 30.0%.
Here PTC has been used for the reaction are tert-Butly ammonium bromide and Benzyl triethylammonium chloride. Both the catalyst have identical yield.
Discussion
10% and 7% Suspended Concentration (SC) Formulation of Potassium 2,4 and 2,6-bis[(4,6-dimethoxypyrimidin-2-yl)oxy]benzoate sample was prepared and tested in rice crop having weeds 2,6 isomer gives good results then 2,4- isomer. The structure of the compound has been confirmed by NMR, IR and LCMS.
References
- N.Wada , Y.Toyokawa , Kumiai Chemical Industry co. ltd., Japan .Pyrimidine derivatives and herbicidal method and composition., 1990 , United States Patent 4906285.
- Yves Bessard* and Roger Crettaz Department of Process Research, Lonza Ltd, P.O. Box CH-3930 Visp, Switzerland Rate Acceleration of Nucleophilic Substitution of 2-Chloro-4,6-dimethoxypyrimidine by Sul®nate Catalysis., Tetrahedron:2000, 56 , 4739 – 4745
- Singh, S., L. Bhushan, J.K. Ladha, R.K., Gupta, A.N. Rao and B. Sivaprasad, 2006. Weed management in dry-seeded rice (Oryzasativa) cultivated on furrow irrigated raised bed planting system. Crop Prot., 25: 487–495.
- Rao, A.N., D.E. Jhonson, B. Sivaprasad, J.K. Ladha and A.M. Mortimer, 2007. Weed management in direct seeded rice. Adv. Agron., 93: 153–255
- Yadav D. B., Yadav Ashok1, Punia S. S.1CCSHAU Regional Research Station, Karnal (Haryana), India.Evaluation of Bispyribac-sodium for Weed Control in Transplanted Rice. Indian Journal of Weed Science 2009 : 41, Issue : 1&2
- Weedicide bispyribac-sodium method for preparing 2012, CN 101265235 B

This work is licensed under a Creative Commons Attribution 4.0 International License.









