Method Development and Validation for the Simultaneous Estimation of Cinitapride and Pantoprazole in Solid Dosage Forms By RP-HPLC
Suryadevara Vidyadhara1*, Yarraguntla Srinivasa Rao2, Anne Ramu1, Reddyvalam Lankapalli Sasidhar1 and Anne Jaya Ramya1
1Chebrolu Hanumaiah Institute of Pharmaceutical Sciences, Chandramoulipuram, Chowdavaram, Guntur-522019, Andhra Pradesh, India. 2Vignan Institute of Pharmaceutical Technology, Duvvada, Visakhapatnam- 530049, A.P
DOI : http://dx.doi.org/10.13005/ojc/290355
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
A new rapid, precise and sensitive reverse phase high performance liquid chromatographic (RP-HPLC) method has been developed and validated for the estimation of cinitapride and pantoprazole simultaneously in combined dosage form. The two components cinitapride and pantoprazole were well resolved on an isocratic C18 column, utilizing a mobile phase composition of acetonitrile: phosphate buffer (50:50, v/v, pH 6.8) at a flow rate of 1.0 mL/min with UV detection at 281 nm. The retention time of cinitapride and pantoprazole were 4.5min and 5.4min respectively. The developed method was validated for specificity, linearity, precision, accuracy, limit of detection (LOD), limit of quantification (LOQ) and robustness as per ICH guidelines. Linearity for cinitapride and pantoprazole were found in the range of 1.5-10.5μg/ml and 20-140μg/ml, respectively. The percentage recoveries for cinitapride and pantoprazole ranged from 97.9-103.44 % and 98.9-103.1%, respectively. The proposed method could be used for routine analysis of cinitapride and pantoprazole in their combined dosage forms.
KEYWORDS:Cinitapride;Pantoprazole;RP-HPLC
Download this article as:| Copy the following to cite this article: Vidyadhara S, Rao Y. S, Ramu A, Sasidhar R. L, Ramya A. J. Method Development and Validation for the Simultaneous Estimation of Cinitapride and Pantoprazole in Solid Dosage Forms By RP-HPLC. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290355 |
| Copy the following to cite this URL: Vidyadhara S, Rao Y. S, Ramu A, Sasidhar R. L, Ramya A. J. Method Development and Validation for the Simultaneous Estimation of Cinitapride and Pantoprazole in Solid Dosage Forms By RP-HPLC. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=372 |
INTRODUCTION
Cinitapride (Figure1A) is a substituted benzamide that acts as a gastro prokinetic and antiulcer agent. It is chemically 4-amino-N-[1-(3-cyclohexen-1-ylmethyl)-4-piperidinyl]-2-ethoxy-5-nitrobenzamide1. Pantoprazole (Figure 1B) is a proton pump inhibitor drug used for short-term treatment of erosion and ulceration of the oesophagus caused by gastro oesophageal reflux disease. Pantoprazole is chemically 5-(Difluoromethoxy)-2-[[(3,4- dimethoxy-2-pyridinyl) methyl [sulfynyl]-1H benzimidazole. It acts by the inhibition of hydrogen-potassium adenosine triphosphatase (H+/ K+ – ATPase) in gastric parietal cells2, 3.
Pantoprazole is official in European Pharmacopoeia4 and Indian Pharmacopoeia5. Cinitapride is not official in any Pharmacopoeia. Literature survey revealed about RP-HPLC methods for individual drugs 6-12. However, RP-HPLC methods employing UV-Visible detection have been reported in combination with other drugs. Hence in the present work a simple precise and accurate, RP-HPLC method was developed for the estimation of cinitapride and pantoprazole in combined dosage forms.
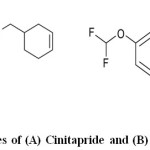 |
Fig 1: Molecular structures of (A) Cinitapride and (B) Pantoprazole Click here to View figure |
MATERIALS AND METHODS
Materials
A reference standard sample of cinitapride was obtained as a gift sample from Avon organics, Hyderabad. Pantoprazole was obtained as a gift sample from Life Line Formulations, Vijayawada. HPLC grade potassium dihydrogen phosphate (KH2PO4), sodium hydroxide, acetonitrile and water were procured from Merck India. For the estimation of commercial formulation, Cintodac capsules having cinitapride 3mg and 40mg pantoprazole manufactured by Cadila health care ltd were procured from the local market.
Instrumentation
Agilent 1120 compact LC chromatographic system, with variable wavelength UV detector and Rheodyne injector equiped with 20 µL fixed loop was used for the chromatographic separation. The chromatogram was recorded at and peaks quantified by means of Ezchrome software. Chromatographic separation was carried out on a C18 column [Agilent ODS UG 5 column, 250mm x 4.5mm 5µ]. AXIS AGN204–PO electronic balance was used for weighing the samples. Ultra-sonic bath sonicator was used for degassing and mixing of the mobile phase.
Chromatographic conditions
Chromatographic separation of cinitapride and pantoprazole was carried on a C18 column. The mobile phase was composed of acetonitrile and phosphate buffer (pH 6.8) in the ratio of 50:50 v/v. It was filtered through a 0.45 μ membrane filter and degassed for 15 minutes. The flow rate of the mobile phase was maintained at 1 ml/min. Detection was carried out at 281 nm at ambient temperature.
METHOD DEVELOPMENT
Preparation of Standard Stock Solutions
Standard stock solutions were prepared by dissolving 25 mg of cinitapride and pantoprazole working standard in two separate 25mL volumetric flasks using 10mL of mobile phase and made up to the mark with mobile phase to obtain a final concentration of 1000 µg/mL of each cinitapride and pantoprazole. From the above stock solutions, 1.5 and 20 ml aliquots each were pipetted in to a 50mL volumetric flask and dissolved in 25mL of the mobile phase and made up to the mark with the solvent to obtain a final concentration of 30µg/ml and 400 µg/mL for cinitapride and pantoprazole respectively. Aliquots of each standard solutions were transferred to a series of 10ml volumetric flasks and the volume was made up to mark with mobile phase to give binary mixtures of various concentrations ie 1.5, 3, 4.5, 6, 7.5, 9, 10.5 μg/mL for cinitapride and 20,40,60,80100, 120,140 μg/mL for pantoprazole
Preparation of Sample solutions
Twenty capsules each containing 40 mg pantoprazole and 3mg cinitapride were taken. A quantity of powder equivalent to 40 mg pantoprazole and 3mg cinitapride was weighed and transferred to a 100ml volumetric flask. 50mL of the mobile phase was added and sonicated for 30 minutes. The solution was made up to the mark with the mobile phase and filtered through a 0.45µ membrane filter. 10 ml aliquot of the above solution was transferred to a 10 ml volumetric flask to obtain a concentration of 30 and 400µg/mL of cinitapride and pantoprazole respectively.
METHOD VALIDATION
The developed HPLC method for the simultaneous determination of cinitapride and pantoprazole was validated as per the ICH guidelines13,14.
System suitability
System suitability for chromatographic separation was checked on each day of validation to evaluate the components of the analytical system in order to show that the performance of the system meet the standards required by the method. System suitability parameters established for the developed method include number of theoretical plates (efficiency), Resolution, Tailing factor. The HPLC system was equilibrated using the initial mobile phase composition, followed by 5 injections of the standard solution of 100% concentration containing 10.5 µg/mL cinitapride and 140 µg/ml pantoprazole. These 5 consecutive injections were used to evaluate the system suitability on each day of method validation. The result was given in the Table 1.
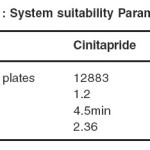 |
Table 1: System suitability Parameters Click here to View table |
Specificity
For determining the specificity of the method, 100% test concentration was injected to the chromatographic system in triplicate. Interferences due to the presence of excipients were not traced in the chromatogram and the chromatographic parameters were not affected. Thus the selected method was specific for the determination of the marketed formulation.
Linearity and range
The linearity was evaluated using external calibration curve with seven calibration levels prepared in triplicate (n=3). Appropriate aliquots were pipetted out from the standard stock solution into a series of 10mL volumetric flasks obtain a concentration range, ranging from 1.5-10.5µg/mL of cinitapride and 20-140µg/mL of pantoprazole. The responses were measured as peak area. The calibration curves were obtained by plotting peak area against concentration .The result of regression analysis was given in the Table 2.
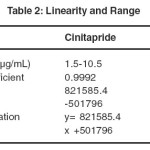 |
Table 2: Linearity and Range Click here to View table |
Accuracy
The accuracy of an analytical method is the closeness of results obtained by that method to the true value for the sample. It is expressed as recovery (%), which is determined by the standard addition method. In the current study recovery at three spike levels 80%, 100% and 120% were carried out. The % recovery at each spike level was calculated and was given in Table 3.
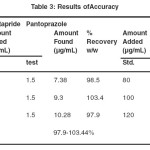 |
Table 3: Results of Accuracy Click here to View table |
Precision
The precision of an analytical method is the closeness of replicate results obtained from analysis of the same homogeneous sample. Precision was considered at different levels, i.e. method, system, interday and intraday. Precision of the developed method was assessed by measuring the response on the same day (intraday precision) and next two consecutive days (inter day precision). The precision of the method was assessed by six replicate injections of 100% test concentration. Intra and inter-day precision of the method was assessed by determination of standard deviation and % RSD for the analyte response. The result was given in Table 4.
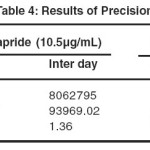 |
Table 4: Results of Precision Click here to View table |
LOD and LOQ
LOD and LOQ values were determined by the formulae LOD = 3.3 σ/S and LOQ = 10 σ/S (Where, σ is the standard deviation of the responses and S is the slope of the calibration curves). In the present method σ is the mean of standard deviation of y intercepts of the three calibration curves and S is the mean of slopes of the calibration curves. The result was given in Table 5.
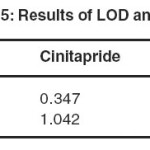 |
Table 5: Results of LOD and LOQ Click here to View table |
Robustness
The robustness of the method was determined by assessing the ability of the developed method to remain unaffected by the small changes in the parameters such as percent organic content, pH of the mobile phase, buffer concentration, temperature, injection volume and flow rate. A deviation of ± 2nm in the detection wavelength, ± 0.1 mL/min in the flow rate, ± 5% change in the organic phase were tried individually. The result was given in the Table 6.
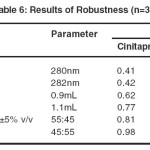 |
Table 6: Results of Robustness (n=3) Click here to View table |
RESULTS AND DISCUSSION
Column chemistry, solvent selectivity, solvent strength (volume fraction of organic solvent(s) in the mobile phase), detection wavelength and flow rate were varied to determine the chromatographic conditions for giving the best separation. Several mobile phase compositions were tried to resolve the peaks of cinitapride and pantoprazole. The optimum optimum results were attained with acetonitrile: phosphate buffer (pH 6.8) in the ratio 50:50(v/v) because it could resolve the peaks of cinitapride with retention time at 4.5 min and pantoprazole retention time at 5.4 min. The two peaks were symmetric and sufficiently resolved.
System suitability was carried out by injecting 5 replicate injections of 100% concentration of cinitapride and pantoprazole. With increment of injection volumes, the tailing factor was less than 1.5 for both cinitapride and pantoprazole. The theoretical plate number was less than 1% and is satisfactory. The resolution was found to be greater than 2 and the other parameters are presented in Table 1.
Specificity of the chromatographic method was tested by injecting sample concentration prepared from marketed formulation. The response was compared with that obtained from the standard drug. The chromatogram confirms the presence of Cinitapride and Pantoprazole at 4.5min and 5.4min respectively without any interference. Thus the developed method was specific for analyzing the commercial formulations for cinitapride and pantoprazole. An optimised chromatogram with the retention times of cinitapride and pantoprazole was shown in the Figure 2.
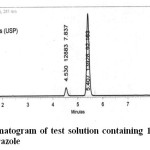 |
Fig2: An optimised chromatogram of test solution containing 10.5 μg/mL of Cinitapride and 140μg/mL of Pantoprazole Click here to View figure |
The peak areas corresponding to the concentration range of cinitapride 1.5 -10.5 µg/mL and pantoprazole 20-140 µg/ml prepared in triplicate were plotted against the respective concentrations. The calibration curves were linear in the range studied for Cinitapride and Pantoprazole, respectively, with mean correlation coefficients (n=3) of 0.999 and higher, the representative calibration curve is shown in Figure 3. The regression analysis was given in Table 2.
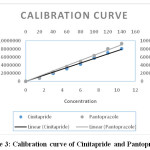 |
Figure 3: Calibration curve of Cinitapride and Pantoprazole Click here to View figure |
Accuracy of the proposed method was assessed by standard addition method at 80%, 100% and 120% levels of recovery to the pre analyzed sample in triplicate. The recovery of the added standard to the sample was calculated and it was found to be 97.9-103.44 %w/w for cinitapride and 98.9-103.1%w/w for pantoprazole respectively and the % RSD was less than 2 for both the drugs which indicates good accuracy of the method. The result of recovery was given in table 3.
LOD and LOQ were calculated from the average slope and standard deviation of y-intercepts of the calibration curve. Limit of detection for cinitapride and pantoprazole were 0.34 µg/mL and 3.38µg/mL respectively where as limit of quantitation of cinitapride and pantoprazole were 1.04 µg/mL and 10.15 µg/mL respectively indicating high sensitivity of the method. LOD and LOQ value was given in table 5. The method is precise with a %RSD of less than 2 for both Cinitapride and Pantoprazole respectively. The results of intraday and inter day precision was given in table 4.
Robustness was carried out by change in the flow rate (±1mL/min), mobile phase variation (±5%) and variation in wavelength (± 2 nm). Solution of 100% concentration is prepared and injected in triplicate for each varied operational condition and % R.S.D was found to be less than 2. The result was given in table 6.
The proposed method was applied for the assay of commercial formulation containing cinitapride and pantoprazole. Each sample was analyzed in triplicate. The mean recovery values were 99.47 and 100.45 for cinitapride and pantoprazole. The result of estimation was given in table 7.
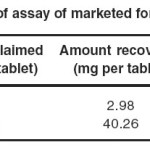 |
Table 7: Results of assay of marketed formulation (n=3) Click here to View table |
CONCLUSION
The proposed RP-HPLC method for simultaneous assay cinitapride and pantoprazole in combined dosage forms was validated, and found to be applicable for routine quantitative analysis of cinitapride and pantoprazole. The results of linearity, precision, accuracy and specificity, were proved to be within the limits. The method provides selective quantification of cinitapride and pantoprazole with no interference from other formulation excipients. Therefore, this method can be employed for the routine analysis for simultaneous estimation cinitapride and pantoprazole in quality control of formulations and also in the dissolution studies.
ACKNOWLEDGEMENTS
The authors are thankful to the management of Chebrolu Hanumaiah Institute of Pharmaceutical Sciences, Guntur, Andhra Pradesh for providing necessary facilities to carry out research work.
REFERENCES
- Connick R. E. and Hugus Z. Z., J. Am. Chem. Soc., 75, 6012(1988).
- Greenstein J.P. and Winitz M., Chemistry of Amino Acids, Vol. II, John Wiley, New York, 1009 (1961)
- The Merk Index. An encyclopedia of chemicals, drugs and biological, 14th edition, Merck Research Laboratories, Division of Merck & Co.USA, (2006)
- Lexi comp’s drug information Hand book international, 13thedition. Charles F. Lacy, (2005).
- Anonymous. Pantoprazole- a third proton pump inhibitor. Drug Ther Bull. 35; 93-4, (1997).
- European Pharmacopoeia, supplement 6.7, Strasburg: European Directorate for the Quality of Medicines and Health Care (EDQM), Council of Europe; (2010).
- Indian Pharmacopoeia, volume 3, Ministry Of Health Family Welfare, The Indian Pharmacopoeia commission; Ghaziabad, (2010).
- Roy S.M.N, Mangoankar K.V., Desai A.Y. and Yetal M. Development and validation of RP-HPLC method for the determination of Cinitapride in pharmaceutical dosage forms., E-journal of chemistry. 7(1): 311-319, (2010).
- Thangabalan.B, Elphine.A Prabahar and Vijay Kumar.P. Validated spectrophotometric estimation of Cinitapride in pure and its solid dosage form. International journal of pharmacy and pharmaceutical sciences. 2 (3). 153-155, (2010).
- Ashok Reddy S, Chandra Shekar KB. and Murali CM. Development and validation of RP-HPLC method to determine Cinitapride hydrogen tartrate in a bulk and pharmaceutical formulation. Journal of global trends in pharmaceutical sciences. 3(2): 619-627, (2012).
- Thangabalan B, Elphine Prabakhar A and Vijay Kumar P. Development and validation of a RP-HPLC method for the determination of Cinitapride in Pharmaceutical dosage forms. Journal of pharmacy research. 4(3): 587-588, (2011)
- Ashour S and Omar S. A modified high-performance liquid chromatographic method for the analysis of pantoprazole sodium in pharmaceutical dosage forms using lansoprazole as internal standard. Arabian Journal of Chemistry. 1-6, (2011)
- Sivakumar.T, Manavalan.R, Muralidharan.C, Valliappan.K. Multi-criteria decision making approach and experimental design as chemometric tools to optimize HPLC separation of domperidone and pantoprazole., Journal of Pharmaceutical and Biomedical Analysis. 43: 1842–1848, (2007)
- SainiV and Gupta VB. Estimation of pantoprazole from multiparticulate dosage form by new HPLC method. Internatinal Journal of PharmTech Research. 1(4): 1094-1096, (009).
- Validation of analytical procedures, methodology Q2 (B) guidelines, 1-10, (2005)
- Text on validation of analytical procedures, ICH Q2 (R1) guidelines, 1-A3, (1995)

This work is licensed under a Creative Commons Attribution 4.0 International License.









