Hydrogels: smart materials for drug delivery
Arti Vashist1, Sharif Ahmad1*
1Materials Research Laboratory, Department of Chemistry, Jamia Millia Islamia, New Delhi
DOI : http://dx.doi.org/10.13005/ojc/290303
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The limitations associated with the conventional therapeutics have intended the use of controlled drug delivery systems. In recent years, the hydrogel technology has been an integral part of human health care. The pharmaceutical industry has been developing hydrogel based drug delivery system in an advanced manner by tuning the structure, shape and surface modifications of the biopolymers. The present review highlights the role of hydrogels in drug delivery. It also highlights the use of important polymers and their applications in drug delivery. In addition, the use of nanocomposite hydrogels with reference to the inorganic and magnetic nanoparticles is also discussed.
KEYWORDS:Hydrogels;drug delivery;biomaterials;starch;nanocomposite
Download this article as:| Copy the following to cite this article: Vashist A, Ahmad S. Hydrogels: smart materials for drug delivery. Orient J Chem 2013;29(3) |
| Copy the following to cite this URL: Vashist A, Ahmad S. Hydrogels: smart materials for drug delivery. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=246 |
Introduction The term hydrogels was originally introduced by Wichterle and Lim in 1960s and its biological application was put forward.1 Since then, hydrogel technology has evolved at a huge scale in pharmaceutical industry. The term hydrogel is self explanatory for their existence, since the evolution of life on earth. The structure of plants, the components of extracellular matrix, the bio-films of microorganisms are everywhere, all the swollen moieties in nature are the proofs of their occurrence. The first paper sighted was by DuPont scientist in 1936 for medical applications, which introduced the spark that was enlightened in 1960 by Wichlerte and Lim who worked on poly (2-hydroxyethylmethacrylate) poly(HEMA).1 It highlighted the properties of this brittle polymer as a highly water swollen, soft and elastic gel. This led to the keen interest in hydrogels as a class of biomaterials and their application as drug delivery systems. The natural and synthetic polymer in demand for the synthesis of DDS is tabulated below. The emphasis in this review is to highlight the few special biopolymers like hydroxyethylcellulose (HEC), Poly (lactic –glycolic acid) (PLGA), starch, NIPAAm (N-isopropylacrylamide). Hydrogels are defined as a three dimensional biopolymeric networks, which have the tendency to absorb large quantity of water and they themselves are not soluble in water. A three dimensional network formation occurs by the cross linking of the polymeric chains. This cross linking can occur via physical interactions, covalent bonding, hydrogen bonding and by van der walls interactions.2 The chemistry behind the interpenetrating hydrogel networks (IPN) can be best understood in terms of the presence of the specific functional groups viz., -OH, -CONH2, -SO3H, -CONH,-COOR which have a hydrophilic tendency and thus absorb water and biological fluids. The soft and rubbery surface, structure and physico-chemical properties of hydrogels mimic to that of human tissue. These characteristic features make them potential candidate for drug delivery systems. Recently, biopolymers have emerged as a smart candidate for the synthesis of hydrogels for drug delivery. Classification of hydrogels The important parameters opted for the classification of hydrogels is the structure of hydrogels, route of synthesis, types of crosslink’s. The differences in the properties are the consequences of the variation in any of the defined characteristics. Firstly, on the basis of route of their synthesis can be classified as:3
- Homopolymer hydrogels (made up of only one type of hydrophilic monomer)
- Copolymer hydrogels or network gels (composed of two types of monomers)
- Mutipolymer hydrogels (made up of three types of monomers or inter penetrating polymeric network)
Secondly the other mode of classification is on the basis of type of ionic charges present on polymer networks:
- Anionic hydrogels (anionic thermoassociative carboxymethylpullulan hydrogels 4
- Cationic hydrogels (new thermosensitive, cationic hydrogels of N-isopropylacrylamide (NIPAM) and (3-acrylamidopropyl)trimethylammonium chloride (AAPTAC) 5
- Neutral hydrogels (miscible blends from water-insoluble polymers like poly(2,4,4-trimethylhexamethylene terephthalamide) 6
- Ampholytic hydrogels (acrylamide based ampholytic hydrogels) 7
Thirdly, on the basis of physical structures hydrogels can be classified as:
- Amorphous hydrogels (chains are randomly arranged)8
- Semi crystalline hydrogels (Dense regions of ordered macromolecular arrangement)9
- Hydrogen bonded or complexation structures (the three dimensional network formed due to Hydrogen bond)10
Emergence of hydrogels as drug delivery system The word smart polymers originated from the ability of hydrogels to imitate the non-linear response of DNA and Proteins.11 Above all the characteristic features of hydrogels, their behaviour to adapt structural changes in response to various physical or chemical trigger (Figure 3) and make them intelligent candidates for drug delivery system. The current drug delivery systems are grievous, ineffective and meddlesome. The discovery of Micro/Nano Hydrogels provided perspicacious means of sustained drug delivery systems that unfold the above obstacles. Furthermore, the drug release kinetics may be reconciled by modifying the shape, size and drug distribution of the hydrogels during assembling process. The smart hydrogels loaded with cancer drugs resulted in sustained release of the drugs until they reach the target cancer cells. These types of systems provide great potential for a safe and effective vehicle for the future drugs with improved mechanisms.12 The hydrogels from natural resources have become an integral part of human health care system.13 Biodegradable and pH responsive hydrogels The advancement in the polymer science has led to the development of effective and smart biodegradable polymeric hydrogels that have the ability to self regulate the drug delivery in response to a specific stimulus. The degradability can be broadly classified in two terms (i) the physical damage also termed as erosion of the polymers, which is dependent on the dissolution and diffusion process, (ii) the chemical degradation.14 The biodegradability of the polymers can be modified by introducing various labile groups such as ester, orthoester, anhydride, carbonate, amide, urea and urethane in their backbone.14 The most valuable characteristics of hydrogel, which make them suitable candidate to be used in drug delivery system is their ability to respond to external stimuli specifically to pH variation. The mechanism behind it can be understood in simple terms. As it is known that the hydrogels are the swollen ionic network containing either acid or basic pendant groups, which can ionize and develop fixed charge on the polymer matrix. All the ionic materials possess a pH and ionic strength sensitivity. The swelling force actually dominates over the non-ionic materials, which are the aftermath of the localization of fixed charges present on the pendant groups. The result is that the total mesh size of the network changes to a large extent with a small variation of pH in the environment.15 Figure 1 demonstrates the swelling and deswelling behaviour of hydrogel networks with the change in temperature and pH. pH responsive nature of hydrogels is studied extensively for drug delivery applications. The structure of the polymeric matrix plays an important role in deciding its pH responsive characteristics. The pendant acidic carboxylic and sulphonic acids or basic functional groups like ammonium salts acts as proton acceptor or donor with the change in pH. Figure 2 shows the structures of the polymers ,which are used in synthesizing the hydrogels for drug delievery systems. Smart polymers used for hydrogel based drug delivery systems Poly (lactic –glycolic acid) The anionic polymerization of this cyclic lactide monomer was performed in early 1960’s. Kulkarni et al. 16 was the first to use this polymer in medicine. The team found that there was no side effect of the implantation of these polymers in guinea pigs and rats and there was a subsequent degradation of the polymer hydrogel matrix. Thus the copolymer of lactic and glycolic acid s widely opted for the controlled release of the pharmaceuticals excepients. Langer and Vacant 1993 were the pioneers who developed these polymers in the form of porous scaffolds in tissue engineering.17 This emerging copolymer, which is being exposed exclusively to be used in the synthesis of various drug delivery systems (DDS), owing to its biocompatibility and biodegradability has been approved by food and drug administration (FDA) for use in biomedical applications. The important parameter for this copolymer is that it shows a glass transition temperature in the range of 40-60 ºC unlike to the homopolymers of lactic acid (polylactide) and glycolic acid (polyglycolide), which show poor solubility. Thus this makes it a suitable candidate to be used in the synthesis of various thermo responsive hydrogels.18 Basically thermo responsive hydrogels are a dispersion, which is in solution form at room temperature and gets converted into a gel on instillation into the human body and give a suitable mode of administration and help in release of the drug. A triblock copolymer Polyethylene glycol-polylactic acid, glycolic acid-polyethylene glycerol (PEG-PLGA-PEG), properties of this copolymer can be altered by varying the ratio of hydrophilic and hydrophobic segments, block length and polydispersity. Lee et al. 19 used this triblock as a wound dressing and scaffold. It was observed that thermo sensitive hydrogel increased the engraftment of muscle derived stem cell (MDSCs) by 20 to 30- fold unit till 20 days. The increased engraftment resulted in enhanced wound healing, which was shown by the wound closure rate, epithelium migration and collagen deposition. Thus it was hypothesized that postnatal (MDSC) in the appropriate environment could regenerate tissue in diabetic wound as well as this system is quite biocompatible with diabetic skin wound and prompted engraftment of stem cells. Similar efforts for the release behaviour for two moral drugs ketoprofen and spironolatone, was studied by Jeong et al. 20 ,which have different hydrophobicities, from the PEG–PLGA–PEG triblockcopolymer hydrogel formed in situ by injecting the solutions into a 37°C aqueous environment. Ketoprofen (a model hydrophilic drug) was released over 2 weeks with a first-order release profile, while spironolactone (a model hydrophobic drug) was released over 2 months with an S-shaped release profile. The release profiles were simulated by models considering degradation and diffusion, and were better described by a model gel. In an evaluation the use of PLGA copolymers and it’s composite with carbon fibers or hydroxyapatite in the regeneration of bone tissue has been seen the mechanical properties of the composite to have great endurance-elasticity relationship in relation to their density. They provide fibrous three dimensional bases and other applications like bone defect restoration in tissue engineering.21 Akdemir et al. 22 also prepared biocompatible and UV cured fumarated poly (ether-ester)-based tissue engineering hydrogels. The modification of the poly (lactide-co-glycolide) (PLGA) copolymer was done using stannous octoate as a catalyst. These hydrogels were found to be biocompatible confirmed by MTT cytotoxicity assay. Hydroxyethylcellulose Hydroxyethyl cellulose is an important derivative of cellulose, which finds application in drug delivery. The highlighting properties of HEC such as, water solubility, non-ionic form , thickening agent, and can form suspension and emulsion adhesion, film formation, and dispersion, water retention, providing protection make this a suitable candidate to be used in synthesis of hydrogels used for drug delivery. Of all these characteristics HEC has water retention is twice of methyl cellulose and hydroxypropyl cellulose, and the protection power for collide is the strongest. This section addresses the new progress in hydroxyethyl cellulose based hydrogels fabrication. The glucose repeat unit is replaced with hydroxyethyl ether. These are the favourable groups that do not allow the polymer to crystallize and when this is added in solution it becomes soluble. Firstly, the nanocomposite hydrogels prepared by using HEC in conjugation with other polymers through physical or chemical blending and the formation of temperature responsive hydrogels based on it and its derivatives and their application is discussed. Gorgieva et al. 23 synthesized a unique hydrogel with dual responsive absorption properties using HEC and carboxymethyl cellulose in an aqueous solution and using citric acid (CA) as a cross-linker. The resulted hydrogel formed showed a swelling profile of knitted cotton fabricated with a thin surface layer of modifying hydrogels was also investigated. In an important investigation by Hoemann et al. 24 prepared cytocompatible chitosan hydrogels crosslinked by HEC-Glyoxal, which were employed for cartilage repair applications. Cell viability and metabolic MTT assay were perfomed and hypothesized that the trace glyoxal present in commercially available HEC is responsible for solidification of Chitosan-GP/HEC hydrogels and these gels can be used as a cytocompatible adhesive delivery vehicle for articular cartilage repair applications. Thus it is seen that HEC can be used for in situ delivery of cells and other factors for a therapeutic effects. The effect of incorporation of nanofillers in HEC matrix was studied by Dai et al. 25 studied the effect of nanofillers like oxidized cellulose nanocrystals and montmorillonite nanoclay on CMC/HEC hydrogels system. Efforts have been made to develop hydrogel membranes using HEC for wounds. Researchers are working on the development of membranes, which is transparent and the wounds can be visualized during the course of dressings without disrupting the healing process. This invention developed hydrogels that can be made from fabrics made from woven or non woven gel forming fibers. These reinforcing materials results into transparent or translucent on adsorption of exudates, which makes easy for the visualization of the wound. The sheet is made up of hydrogel polymer. The removal of the dressing is exempted by this invention. In particular, derivatives of cellulose, which are gel forming fibres with absorbency of between 10g/g of sodium / calcium chloride, are used. Formulations of many vaginal microbicides constitute HEC as an inactive ingredient. These microbicides are designed to prevent the transmission of sexually transmitted diseases, including HIV. This polymer has been studied for use as a placebo gel in clinical trials of HIV microbicides. Its use as a universal placebo for HIV microbicide trials has been adopted and the safety of this product is being evaluated.26 A study reports the use of nimesulide topical gels using this natural bioadhesive polymer HEC and it was found that effect of polymer on bioadhesive strength was extremely significant. The drug studied in the present work is a second generation non-steroidal anti-inflammatory drug used for long term therapy of rheumatoid arthritis, in alleviating pain and inflammation. The half life of 3 to 4 h requires multiple dosing for the maintenance of the therapeutic effect in a day, which results in more fluctuation. Thus this gel prevents these side effects in a day, which results in more fluctuation. Thus this provides advantage of delivering the drug into site of action. The reduction in the cost of the therapy and patient compliance are the valuable benefits of this mode of drug delivery.
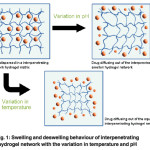 |
Figure 1 Swelling and deswelling behaviour of interpenetrating hydrogel network with the variation in temperature and pH. Click here to View figure |
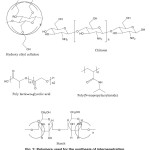 |
Figure 2 Polymers used for the synthesis of interpenetrating hydrogel network used for drug delivery systems. Click here to View figure |
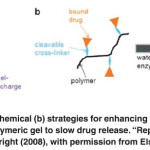 |
Figure. 3 Physical (a) and chemical (b) strategies for enhancing the interactions between a loaded drug and a polymeric gel to slow drug release. “Reprinted from reference 1, Copyright (2008), with permission from Elsevier. Click here to View figure |
Starch Starch is high value natural polymeric material extracted from seeds, tubers or roots and is a chain of glucose molecules by glycocidic linkages. The two forms are amylase (linear) and other is amylopectins (branched). Hydrogels based on starch as base or modifying material represent a great choice in tissue engineering, drug delivery, and other biomedical applications. Recently, Lima et al.27 developed a stimuli responsive injectable hydrogels of chitosan – starch. The research group encapsulated the chitosan –starch based gels derived stromal cells for the regeneration of articular cartilage. The incorporation of the starch gave a firm textured gels and thus providing the desirable mechanical strength. The degradation profile of the gels was also improved. The influence of the inclusion of starch in the chondrogenesis of encapsulated adipose stromal (ADSC) cells was studied. Thus it was found that these chitosan β-glycerophosphate-starch hydrogels can be used for chondrogenic differentiation of ADSC for cartilage regeneration. Starch hydrogels have found their use in wound dressing. Pal et al. 28 attempted to prepare transparent starch based hydrogel membranes by cross linking of polyvinylalcohol with heat treated corn starch suspension. The diffusion coefficient of a fourth generation fluoroquinolone-gatifloxacin was determined to be 3.24 × 10‑6 cm2/s techniques with biocompatibility towards L929 fibroblast cell. NANOCOMPOSITE HYDROGELS Nanocomposite hydrogel are defined as cross linked three dimensional water swollen networks in the presence of nanoparticles. The formation of the network occurs by both physical and chemical interactions. The inclusion of nanoparticles in the hydrogel matrix add on to the unique physico-chemical characteristic properties to hydrogels like mechanical, optical, thermal, barrier, sound, electric simulations etc. 29 Nanocomposite based on inorganic and magnetic nanoparticles The property of aggregation is shown by nanoparticles, which are neither charged nor stabilized. Exfoliation in water may occur for charged nanoparticles such as silicates due to the colloidal interactions that stabilize the gel formed. For the formation of stabilized hydrogel nanocomposite the dispersion of these nanopaticles should be uniform and the formation of large scales structure need to be controlled. Thus, the addition of silicates to poly (ethylene oxide) and poly (acryl amide) matrices function as cross-linking agents and to improve network strength. Researchers found that hydrogen bonding, ionic, dipole, and other interactions such as polymer entanglements must play a role in typical cross linking. A large body of literature and some recent reviews covers the synthesis, characterization, and applications of polymer nanocomposite hydrogels containing inorganic and magnetic nanoparticles.30, 31 Takahashi et al. 32 has shown that a modified PEO–Laponite system can be developed into a drug delivery system at physiological conditions. A broader variety of applications is mentioned for colloidal dispersions (attractive gels) made from nanoparticulate bentonites (natural layered silicate) and PEO polymer. Inclusion of metal nanoparticles (i.e., Ag, Au) dispersed within polymer hydrogels can improve electrical conductance and anti-microbial properties 33, 34. Nanoparticles can also function as cross-linking (physical and chemical) agents. Polymer–magnetic nanocomposites, with particles in the polymer matrix (dispersed within and/or cross linking polymer chains), can be used for the remote release of drugs.35, 36 Negatively charged silica nanoparticles are immobilized within a PAM matrix. An applied electric field causes electro-osmotic flow of silica particles, and viscous drag within the fluid results in the mass transport of neutral solutes (drugs, proteins, etc.) smaller than the gel pore size. 29 F Templated block-copolymer gel with nanoparticles residing in the interstitial space between neighbouring micelles.37 Recently, the significance of kaolinite on the mechanical properties of PVA was studied.38 The nanocomposite formed from the inclusion of kaolinite in PVA matrix resulted in a composite of high tensile strength and tensile modulus. The DMTA test was performed in the compression mode, using disc-shaped samples with a diameter of 11 mm and a thickness of 5 mm. The storage modulus of PVA hydrogel increases by increasing the kaolinite content. The storage modulus of nanocomposite hydrogel containing 15 wt.% of kaolinite in the regions below and above 0ºC were on average 210 and 140 % higher than of pure PVA hydrogel, respectively. The results also showed that the hardness is directly depended to the quantity of kaolinite added to the nanocomposite hydrogel.38 Nanocomposite hydrogels for biomedical applications have been studied. A research group prepared poly(hydroxyethyl acrylate), PHEA/silica nanocomposites by sol-gel techniques as potential scaffold materials for tissue engineering.39
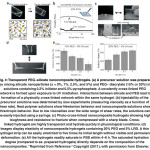 |
Figure 4 Transparent PEG–silicate nanocomposite hydrogels. (a) A precursor solution was prepared by mixing silicate nanoparticles … Click here to View figure |
In an interesting study carried out by Gaharwar et al.40 hydrogels composed of PEG and silicate nanoparticles (Laponite RD) were investigated. This study demonstrated the potential of silicate –PEG nanocomposite hydrogels in orthopaedic, craniofacial sand dental applications. Figure. 4 (a-b) showed viscosity measurements as a function of shear rates. The results showed the potential of the synthesized nanocomposite to retain the shape of the mold containing them (Fig. 4) and were capable to sustain the extreme mechanical deformation. Moreover, the amount of silicate nanoparticles affected the swelling characteristics of the nanocomposites. Several other aspects of polymer nanocomposites are currently being intensively investigated, such as the effects of nanofiller on chain dynamics, and the dependence of the final properties on the degree and mode of dispersion Novel hydrogels have been prepared using direct dissolution of chitin at low temperature. The dissolution problem is common for chitin in various solvents. Clear transparent solution in a mixture of 8wt%NaOH/ 4wt% urea aqueous solution by freeze /thawing method to prepare transparent.41 Furthermore, the results of a 293T cell viability assay on this system indicated their excellent biocompatibility and safety. Thus resulted chitin hydrogels showed more stable structure and better biocompatibility. The chitin hydrogels may find wide use in bio-applications, as a result of the more stable structure and better compatibility of chitin than that of its derivatives, such as chitosan.41 Hydrogel nanocomposites are also being studied as pressure-sensitive adhesives for skin contact applications. The effects of monodisperse polystyrene nanoparticle fillers on the network formation, rheological properties and adhesion performance of hydrogel nanocomposites based on polyacrylamide and poly(acrylamide-hydroxyethyl methacrylate) is investigated.42 Organ clay is an organically tailored phyllosilicate, derived from a naturally occurring clay mineral. The incorporation of these organo-clays in Polydimethyl siloxane (PDMS) results in formation of pressure sensitive adhesives. Shaikh et al. 43 obtained the partially exfoliated nanocomposites of PDMS. It was shown that by varying the concentration of the organoisilicate additive to the polymer matrix, a major control over the drug release kinetics and the adhesive properties of the matrix can be enhanced. Now days the emphasis is to develop transdermal formulations, which are based on natural polymer matrix and for better release technologies in, which the dispersion of the drug is uniform within the transdermal layer.44, 45 Future prospectives and conclusion The innovation behind the successful application of hydrogel based drug delievery system is the development of new polymeric materials and advancement in nanotechnology. The key to improve the hydrogel technology is to direct the research on the design of efficient drug delievery systems with minimal limitations and easy route of administrations. The use of biodegradable synthetic polymers have shown prominent results in drug delievery. Self assembled hydrogel and hydrogels for tumor targeting and imaging are to be explored at a greater pace. The valuable addition of an efficient drug delievery systems in comparision to the development of newly found drug can benefit both ecnomically as well as drastically reduce the duration of time taken to develop a new drug in the pharmaceutical world. References
- O. Wichterle and D. Lim, 1960.
- Y. Qiu and K. Park, Advanced Drug Delivery Reviews, 2001, 53, 321-339.
- N. Peppas, P. Bures, W. Leobandung and H. Ichikawa, European journal of pharmaceutics and biopharmaceutics, 2000, 50, 27-46.
- G. Mocanu, D. Mihaï, V. Dulong, L. Picton and D. Le Cerf, Carbohydrate Polymers, 2012, 87, 1440-1446.
- N. Deo, S. Ruetsch, K. Ramaprasad and Y. Kamath, Journal of cosmetic science, 2010, 61, 421.
- V. Percec, T. K. Bera and R. J. Butera, Biomacromolecules, 2002, 3, 272-279.
- J. P. Baker, H. W. Blanch and J. M. Prausnitz, Polymer, 1995, 36, 1061-1069.
- S. Bale, V. Banks, S. Haglestein and K. G. Harding, J Wound Care, 1998, 7, 65-68.
- R. F. Ofstead and C. Posner, Polymers in Aqueous Media. Washington, DC: American Chemical Society, 1989, 61-72.
- X. Li, W. Wu and W. Liu, Carbohydrate Polymers, 2008, 71, 394-402.
- I. Y. Galaev and B. Mattiasson, Trends in biotechnology, 1999, 17, 335-340.
- H. T. Ta, C. R. Dass and D. E. Dunstan, Journal of Controlled Release, 2008, 126, 205-216.
- Y. Zhang, Y. Han, Z. Chu, S. He, J. Zhang and Y. Feng, Journal of Colloid and Interface Science, 2013, 394, 319-328.
- A. Göpferich, Biomaterials, 1996, 17, 103-114.
- N. Peppas and A. R. Khare, Adv. Drug Delivery Rev., 1993, 11, 1-35.
- R. K. Kulkarni, K. C. Pani, C. Neuman and F. Leonard, Arch Surg, 1966, 93, 839-843.
- A. G. Mikos, G. Sarakinos, S. M. Leite, J. P. Vacant and R. Langer, Biomaterials, 1993, 14, 323-330.
- W. S. Shim, J.-H. Kim, H. Park, K. Kim, I. Chan Kwon and D. S. Lee, Biomaterials, 2006, 27, 5178-5185.
- P. Y. Lee, E. Cobain, J. Huard and L. Huang, Molecular Therapy, 2007, 15, 1189-1194.
- B. Jeong, Y. H. Bae, D. S. Lee and S. W. Kim, Nature, 1997, 388, 860-862.
- R. C. Thomson, M. J. Yaszemski, J. M. Powers and A. G. Mikos, Biomaterials, 1998, 19, 1935-1943.
- Z. S. Akdemir, N. Kayaman-Apohan, M. V. Kahraman, S. E. Kuruca, A. Güngör and S. Karadenizli, Journal of Biomaterials Science, Polymer Edition, 2011, 22, 857-872.
- S. Gorgieva and V. Kokol, Carbohydrate Polymers, 2011, 85, 664-673.
- C. Hoemann, A. Chenite, M. Buschmann, A. Serreqi and J. Sun, Google Patents, 2009.
- Q. Dai and J. F. Kadla, Journal of applied polymer science, 2009, 114, 1664-1669.
- D. Tien, R. L. Schnaare, F. Kang, G. Cohl, T. J. McCormick, T. R. Moench, G. Doncel, K. Watson, R. W. Buckheit Jr and M. G. Lewis, AIDS Research & Human Retroviruses, 2005, 21, 845-853.
- H. Sá-Lima, S. G. Caridade, J. F. Mano and R. L. Reis, Soft Matter, 2010, 6, 5184-5195.
- K. Pal, A. Banthia and D. Majumdar, Trends Biomater Artif Organs, 2006, 20, 59-67.
- P. Schexnailder and G. Schmidt, Colloid and Polymer Science, 2009, 287, 1-11.
- A. Razmjou, M. R. Barati, G. P. Simon, K. Suzuki and H. Wang, Environmental science & technology, 2013.
- Y. Hou, A. R. Matthews, A. M. Smitherman, A. S. Bulick, M. S. Hahn, H. Hou, A. Han and M. A. Grunlan, Biomaterials, 2008, 29, 3175-3184.
- T. Takahashi, Y. Yamada, K. Kataoka and Y. Nagasaki, Journal of Controlled Release, 2005, 107, 408-416.
- Y. Murali Mohan, K. Lee, T. Premkumar and K. E. Geckeler, Polymer, 2007, 48, 158-164.
- V. Kozlovskaya, E. Kharlampieva, B. P. Khanal, P. Manna, E. R. Zubarev and V. V. Tsukruk, Chemistry of materials, 2008, 20, 7474-7485.
- N. S. Satarkar and J. Zach Hilt, Acta Biomaterialia, 2008, 4, 11-16.
- T.-Y. Liu, S.-H. Hu, K.-H. Liu, D.-M. Liu and S.-Y. Chen, Journal of Controlled Release, 2008, 126, 228-236.
- D. C. Pozzo and L. M. Walker, Macromolecules, 2007, 40, 5801-5811.
- M. Sirousazar, M. Kokabi, A. Bahramian and Z. Hassan, Advanced Materials Research, 2012, 383, 3854-3857.
- D. Fragiadakis, P. Pissis and L. Bokobza, Polymer, 2005, 46, 6001-6008.
- A. K. Gaharwar, C. P. Rivera, C.-J. Wu and G. Schmidt, Acta Biomaterialia, 2011, 7, 4139-4148.
- C. Chang, S. Chen and L. Zhang, Journal of Materials Chemistry, 2011, 21, 3865-3871.
- N. Bait, B. Grassl, C. Derail and A. Benaboura, Soft Matter, 2011, 7, 2025-2032.
- S. Shaikh, A. Birdi, S. Qutubuddin, E. Lakatosh and H. Baskaran, Annals of biomedical engineering, 2007, 35, 2130-2137.
- M. B. Brown, G. P. Martin, S. A. Jones and F. K. Akomeah, Drug delivery, 2006, 13, 175-187.
- A. Naik, Y. N. Kalia and R. H. Guy, Pharmaceutical science & technology today, 2000, 3, 318-326.

This work is licensed under a Creative Commons Attribution 4.0 International License.









