Kinetics of Oxidation of D-Galactose by Pyridinium Chlorochromate
J. V. Singh1*, Anupam Awasthi1, Dipti2, Ashish Tomar2 and G. L. Agrawal3
1Department of Chemistry, Nehru College, Chhibramau - 209 721, India.
2Department of Chemistry, Meerut College, Meerut - 250 001, India.
3Department of Chemistry, Dr. H.S. Gour Vishwavidyalaya, Sagar - 470 003, India.
The oxidation kinetics of D-Galactose with pyridinium chlorochromate (PCC), have been studied in presence of perchloric acid. The reaction under pseudo-first order condition, is first order with respect to both the oxidant and the substrate. The reaction is markedly catalysed by H+ ions and the effect of [NaClO4] is negligible. A 1:1 stoichiometry is observed. Activation parameters have been computed by measuring the rates at different temperatures. A probable mechanism of the reaction is suggested.
KEYWORDS:Oxidation; Kinetics; Mechanism; D-Galactose; Pyridinium chlorochromate (PCC)
Download this article as:| Copy the following to cite this article: Singh J. V, Awasthi A, Dipti, Tomar A, Agrawal G. L. Kinetics of Oxidation of D-Galactose by Pyridinium Chlorochromate. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Singh J. V, Awasthi A, Dipti, Tomar A, Agrawal G. L. Kinetics of Oxidation of D-Galactose by Pyridinium Chlorochromate. Available from: http://www.orientjchem.org/?p=22995 |
Introduction
In recent years a number of studies on oxidation reactions involving Cr(VI) based oxidants and organic compounds have been made1,2, The oxidation reaction of carbohydrates is complex because of their multifunctional nature. Earlier3,4 and recent5 reviews on the subject are noteworthy. D-Galactose plays an important role in carbohydrate chemistry because it is a constituent of glycolipids and glycoproteins. Our interest6,7 in the use of modified Cr(VI) reagents promoted us to investigate the oxidation of D-Galactose with PCC to throw light on the mechanistic aspects of the oxidation process.
Material and Method
Pyridinium chlorochromate was synthesized by the reported procedure8. Aqueous solution of D-Galactose (Loba-chemie) was always freshly prepared. Perchloric acid and other chemicals (A.R., B.D.H.) were used as such without further purification. The reactions were performed under pseudo-first order conditions by keeping a large excess of D-Galactose with respect to PCC. Kinetic measurements were made using a systronics spectrophotometer 106 at 400 nm. The optical density was measured at various intervals of time.
Stoichiometry and Product Analysis
Stoichiometry of the reaction was observed as one and may be represented as:
C6H12O6 + C6H5NHCrO3Cl → C5H10O5 + HCOOH + C6H5NHCl + CrO2
D-Galactose PCC D-lyxose formic acid
The end product D-lyxose was confirmed by osazone formation9 and by paper chromatography10. The presence of formic acid was confirmed by spot test11.
Result and Discussion
The pseudo-first order rate constants were determined at various initial concentrations of reactants. The results obtained are given in Table 1. Plots for different concentrations of PCC versus time were linear and the rate constants were independent of initial [PCC], showing first order dependence on [PCC].The reaction is first order with respect to D-Galactose also. A plot of log k against [D-Galactose] was linear with a slope of unity, thereby confirming first order dependence in [D-Galactose]. Rates of oxidation were found to increase with increase in [H+] and slopes of the plots of log k, versus log [HClO4] was approximately unity, showing that the reaction is acid-catalysed and follows a first order dependence in [HClO4].
Table 1: Rate Constants for oxidation of D-Galactose by PCC at 30oC
|
[PCC] x 103 |
[D-Galactose] x 102 |
[H+] x 100 |
k1 x 104 |
k2 x 102 |
|
(mol dm-3) |
(mol dm-3) |
(mol dm-3) 1s-1)
|
(s-1)
|
(dm3mol–
|
|
0.40 |
0.40 |
2.32 |
2.18 |
|
|
0.60 |
0.40 |
2.32 |
2.18 |
|
|
0.80 |
0.40 |
2.2 |
2.20 |
|
|
1.00 |
0.40 |
2.32 |
2.20 |
|
|
1.20 |
0.40 |
2.32 |
2.23 |
|
|
0.40 |
0.40 |
2.32 |
2.18 |
5.45 |
|
0.40 |
0.80 |
2.32 |
4.37 |
5.46 |
|
0.40 |
1.20 |
2.32 |
6.55 |
5.46 |
|
0.40 |
1.60 |
2.32 |
8.73 |
5.46 |
|
0.40 |
2.00 |
2.32 |
10.75 |
5.34 |
|
0.40 |
0.40 |
1.74 |
1.64 |
|
|
0.40 |
0.40 |
2.32 |
2.18 |
|
|
0.40 |
0.40 |
2.90 |
2.72 |
|
|
0.40 |
0.40 |
3.48 |
3.28 |
|
|
0.40 |
0.40 |
4.06 |
3.82 |
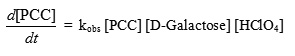
The catalysis by HClO4 suggests that protonated form of PCC is involved in the oxidation. Consequently the empirical rate law is described as follows- The reaction rate was not influenced by ionic strength when NaClO4 was initially added to the reaction mixture over the range of 0.010 to 0.030 mol dm-3. Similar observations were also reported in the oxidation of diols by PCC12.
The reaction rates at different temperatures were determined and the value of activation parameters were calculated from the slope of linear plot of log k versus T-1. The data are presented in Table 2. The entropies of activation is largely negative as expected for bimolecular reaction.
Table 2:Temperature dependence and activation parameters of the oxidation of D-Galactose by PCC.
[PCC] = 0.40 x 10-3 mol dm-3 , [D-Galactose] = 0.40 x 10-2 mol dm-3 , [H+] = 2.32 x 100 mol dm-3, Solvent: Acetic acid-water(50-50 % v/v)
|
Temperature |
k1 x 104 | Ea | ΔH# | ΔG# | -ΔS# |
| (oC) | (s-1) | (kJ mol-1) | (kJ mol-1) | (kJ mol-1) | (JK-1 mol-1) |
| 30 | 2.18 | – | 60.16 | 95.50 | 116.65 |
| 35 | 2.96 | 60.92 | 60.12 | 96.34 | 117.60 |
| 40 | 4.72 | 62.42 | 60.08 | 96.73 | 117.10 |
| 45 | 6.37 | 64.70 | 60.04 | 97.52 | 117.88 |
| 50 | 10.19 | – | 60.00 | 97.84 | 117.15 |
The pyranoid form in its chair conformation is the stable form (4C1) of aldohexoses13,14 and predominantly exists in solution15. In the equilibrium of anomeric form of D-Galactose it is the β-galactose with equatorial orientation of glycosidic hydroxyl group that predominantly exist (scheme 1) and is supposed to be more reactive species16.
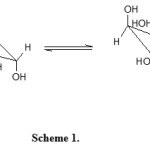 |
Scheme 1 Click here to View scheme |
A free radical mechanism is ruled out since polymerisation of acrylonitriles was not observed. UV spectra did not show existence of the intermediate complex in the present study, however its formation in small amount cannot be ruled out in view of product identification. Hence in the present mechanism it is proposed that a chromate ester is formed in a rapid pre-equilibrium step. The proposed mechanism is outlined in scheme 2.
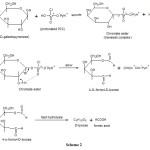 |
Scheme 2 Click here to view scheme |
References
- Mahanti M.K. and Banerji K.K., J. Indian Chem. Soc., 79, 31(2002).
- Patel Sabita and Mishra B.K., Tetrahedron, 63, 4367(2007).
- Capon B., Chem. Rev. 69, 407(1969).
- Bhatnagar R.P. and Fadnis A.G, J. Scient. Ind. Res., 45, 90(1986).
- Varela Oscar, Advances in Carbohydrate Chemistry and Biochemistry, 58, 307(2003).
- Singh J.V., Awasthi Anupam, Mishra K., Agrawal G.L., Pandey A., Oxid. Commun., Communicated(2009).
- Singh J.V., Kumar A, Srivastava K., Mishra K., Pandey A, Agrawal G.L, Oxid. Commun., 27(4), 849(2004).
- Corey E.J and Suggs J.W., Tetrahedron Lett , 2647(1975).
- Vogel A.I “ Practical Organic Chemistry” 2nd Edn., Longman, London(1951) p.442.
- Jermyn and Isherwood, Biochem. J., , 44, 402(1949).
- Feigl F., Spot Test in Organic Analysis, 5th Edn., Elsevier, NY (1956) p340.
- Agrawal G.L. and Jha S., Revue Roumaine de Chimie, , 34, 1769(1989).
- Synder J.R. and Serianni A.S., Carbohydr. Res., , 210, 21(1991).
- Angyal S.J., Adv. Carbohydr. Chem. Biochem., , 49, 24(1991).
- Rudrum M. and Shaw D.F, J. Chem. Soc., 52(1965).
- Perlin A.S., Can. J. Chem., 42, 2365(1964).

This work is licensed under a Creative Commons Attribution 4.0 International License.









