Keto- Enol Tautomerism Of 2-Hydroxy Naphthylideneaniline With Lanthanide Shift Reagent Pr(Fod)3 In Different Solvents
L. M. N. Saleem* and R.H. Sultan
Department of Chemistry, College of Education, Mosul, Iraq
Addition of Lanthanide shift reagents Pr(fod)3 to 2-hydroxynaphthylideneaniline in non polar solvents and slightly polar solvent showed for the first time a keto-enol tautomerism. KT values= ([keto]/[enol]) were calculated in the chosen solvents. KT at fixed con centration of the shift reagent was determined at different temperatures and the thermodynamic parameters (?H0,??G0, ?S0) were obtained.
KEYWORDS:Keto-enol tautomerism; 2-Hydroxy naphthylideneaniline; Lanthanide shift reagents effect
Download this article as:| Copy the following to cite this article: L.M.N. Saleem L. M. N, Sultan R. H. Keto- Enol Tautomerism Of 2-Hydroxy Naphthylideneaniline With Lanthanide Shift Reagent Pr(Fod)3 In Different Solvents. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: L.M.N. Saleem L. M. N, Sultan R. H. Keto- Enol Tautomerism Of 2-Hydroxy Naphthylideneaniline With Lanthanide Shift Reagent Pr(Fod)3 In Different Solvents. Available from: http://www.orientjchem.org/?p=22914 |
Introduction
Aldamines derived from the aromatic aldehydes having a hydroxyl group in the ortho position to aldehyde group are of interest mainly due to existence of either O-H—N or O—H-N type of intramolecular hydrogen bond and the related tautomerism between such enol-imine and keto-amine forms.(1-4)
The electron withdrawing or donating ability of the N-substitunts, their position and stereochemistry, as well as hydrogen bond donor-acceptor properties, can stabilize one or another tautomer in the crystal.(2,5)
The spectra of different schiff-bases have been studied in polar and non-polar solvents and mixed solvents, and it was established that the tautomeric equilibrium in 2-hdroxynaphthylideneaniline schiff-bases depend on the polarity of the solvent, the acidity of the medium, the temperature, and the strength of the hydrogen bond.(6-8)
Theoretical studies of tautomeric equilibrium in orhto-hydroxy schiff-bases have been reported. (9-11)
Earlier (12-14) we have reported the cis-trans isomerization of benzylideneaniline on addition of .
In this communication we would like to present the effect of the lanthanide shift reagent on the UV spectra of the schiff base 2-hdroxy naphthylideneaniline (HNA) in different solvents.
Experimental
Materials
Lanthanide shift reagent, , 99.9% was purchased from Fluka.
Acetylacetone
Solvents: n-heptane, Cyclohexane, Carbontetrachloride, chloro benzene, acetonitrile, toluene, chloroform, dichloromethane, ethanol and DMSO we purified (when required) by distillation at their boiling points.
Preparation of the Schiff-base 2-OH naphthylideneaniline
(0.172 gm) 10-3 M of 2-hydroxy naphthaldehyde (HNA) was dissolved in absolute ethanol (10 ml), to this solution (0.093 gm)10-3 M of aniline (which was freshly distilled) in absolute ethanol was added dropwise with continuous stirring. The mixture was refluxed for 2 hr then was left to cool, the precipitate was separated and dried then recrystallized from ethanol/H2O to yield yellow crystals (m.p= 94-95˚). IR of the product show a sharp band (1621 cm-1, C=N) and a broad band (3444 cm-1, O-H) .
Preparation of solutions
Stock solutions of (5*10-5)M of (HNA) and (5*10-4)M of each was prepared in the mentioned solvents. 2ml of (HNA) in the suitable solvent was placed in a silica cell (1*1*3*cm) and 2ml of the same solvent was placed in the blank cell. Several additions (100-μl) of were performed on the sample cell and the blank cell. The absorption spectra after each addition at room temperature and at higher temperatures (10-50˚) were recorded on shimadzu UV-1800 spectrophotometer equipped with a water bath SB-11 EYRLNTT.
Results and Discussions
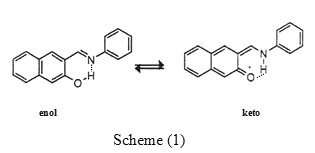
The UV-spectra of (5*10-5 )M (HNA) show two bands the first was located at low wave length related to the enol from, the second located to the high wave length is due to the keto from. The information about the absorbance and wave length of these bands in the absence of are presented in (Table-1).Additions of the lanthanide shift reagent 5*10-4)M in the suitable solvent to the solution of the schiff-base (HNA) in the same solvent resulted in a decrease in the main (enol) band accompanied by an increase in the keto band with the formation of an isobestic point
Fig.1(a-d). At this point an equilibrium between the two forms occur. The isobestic point in the four solvents: n-heptane, cyclohexane, CCl4, and C6H5Cl were observed at λ=411,411,412 and 420 nm, and at absorbance=0.22 ,0.4 ,0.22 and 0.35 respectively.
Non polar solvents have been chosen in this study for two reasons: first because the % keto from in the absence of LSR was too small (Table-1) especially in n-heptane and cyclohexane so that the main band observed in the spectrum is related to the enol, and secondly: in order to observe the effect of in the absence of the factor of polarity.
KT values (KT=[keto]/[enol]), were calculated for (HNA) in four solvents (Table 2) and were found to be: 1.96, 1.942, 1.53 & 5.476 in n-heptane, Cyclohexane, CCl4 and chlorobenzene respectively. These values are calculated under the same conditions of the concentrations of the donor (HNA) and the acceptor and at the same temperature.
It can be seen that KT values in n-heptane and cyclohexane are very close. However in CCl4, although its dielectric constant is near to that for the above two solvents, its KT value is slightly lower which could be attributed to the solvation(7,13) which effect the proton transfer from enol-imine form to the keto-enamine leading to lower the effect of on the process of tautomerism.
In chlorobenzene however, a higher value of KT was observed (5.45). If we go back to the UV-Spectrum of (HNA) in this solvent in the absence of (Fig 1-d) we can notice that the absorbance of the keto band is higher than that observed in the other solvents (see-Table-1) this may be attributed to the slight polarity of this solvent, i.e. we have already started with a high ratio (25%) of the keto, this is added to the effect of .
 |
Fig1: The U.V. Spectra of (5*10-5 )M (HNA) in Click here to View figure |
a: n-heptane b: cyclo hexane c: CCl4 d: dichlorobenzene
1: in the absence of
2,3,4: after additions of 100μl of (5*10)-4 M
Table 1: The spectroscopic values of the keto and enol forms bands of 2-OH-naphthylidene aniline in different solvents.
|
Solvents |
λ nm |
Abs |
%enol |
λ nm |
Abs |
%keto |
|
n-heptane |
379 |
0.651 |
90.54 |
440 |
0.068 |
9.46 |
|
Cyclohexene |
376 |
1.042 |
91.56 |
441 |
0.096 |
8.43 |
|
Carbon tetra chloride |
379 |
0.796 |
81.97 |
436 |
0.175 |
18.02 |
|
Chloro benzene |
380 |
0.699 |
74.76 |
439 |
0.236 |
25.24 |
The UV-spectra were also studied at a fixed concentration of at temperatures ranging between (283-323k˚) in the different solvents. On increasing temperature the keto band decrease, while the enol band increases i.e. the spectrum nearly returns back to its origin. KT values at different temperatures were calculated and were given in (Table 2). The thermodynamic parameters are calculated and were summarized in (Table 3).
Table 2: KT values of 2-OH-naphthylidene aniline in different solvents and at different temperatures (K˚)
|
Solvents |
ε* |
KT |
||||
|
283 |
293 |
303 |
313 |
323 |
||
|
n-heptane |
1.92 |
1.96 |
1.42 |
0.73 |
0.32 |
0.21 |
|
Cyclohexene |
2.02 |
1.94 |
1.29 |
1.01 |
0.69 |
0.57 |
|
Carbon tetra chloride |
2.24 |
1.53 |
0.69 |
0.42 |
0.30 |
0.16 |
|
Chloro benzene |
5.62 |
5.48 |
4.17 |
2.43 |
1.15 |
0.75 |
: dielectric constant
Table 3: The thermodynamic parameters of (HNA) in different solvents.
|
Solvents |
∆H KJ.mol-1 |
∆G KJ.mol-1 |
∆S J.mol-1.K-1 |
|
n-heptane |
45.49 |
1.11 |
150 |
|
Cyclohexene |
39.96 |
-1.99 |
140 |
|
Carbon tetra chloride |
45.54 |
2.20 |
140 |
|
Chloro benzene |
23.37 |
0.27 |
80 |
∆S values are positive which means that the reactants are more ordered than the products indicating that the electrostatic force which accumulate the solvent molecules to the reactact are more than to the product,and due to the intramolecular hydrogen bonding which stabilizes the enol form (scheme-1) .
∆G values are negative in cyclohexane and have low values in the other solvents this indicates that the energy difference between the keto and enol forms is small which explains the easiness of the change to keto on adding and the return back to the enol on increasing the temperatures.
The above results show that tautomerism occur by the effect of indicated from the following points:
No new band was observed in the spectrum to indicate the formation of a complex.
When raising the temperature the keto tautomer decreases with increasing the enol and the compound returns back to its origin with no change in the shape of the bands. This may be due to the decrease in the polarity of the solvent as the temperature increases which prefer the less polar enol form since the keto form is more likely to present in polar solvents(9).
These two points confirm that tautomerization is accelerated by , the process can be abbreviated by:
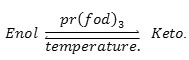
The U.V spectra of (HNA) in solvents having different polarities have been studied (Fig. 2), it can be seen that the absorbance of the keto band is proportional to the polarity of each solvent, suggesting that the proton donor acceptor behavior of the polar solvents in the absence of play a significant role in increasing the keto tautomeric percentage through hydrogen bonding(9).
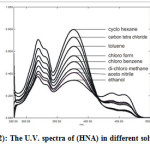 |
Figure 2: The U.V. spectra of (HNA) in different solvents |
However when is added to (HNA) in these polar solvents under the same conditions, no isobestic point was observed but just a decrease in the absorbance of the whole spectrum was observed. This may be because polar solvents interact with thus its effect on the tautomerism process disappears.
In order to through light on the role of the lewis acid on the keto –enol tautomerism observed in the present experiments were performed where two different acids namely CH3COOH and formic acid are used instead of . The effect of acidity on the keto-enol tautomerism is well known(4).This effect was observed in this work by adding several amounts of the acids to the Schiff base (HNA) in cyclohexane it was observed that tautomerism towards the keto formation occurs as the amount of acid increases with the formation of isobestic point (Fig.3). This may in a similar manner explains the effect of acidity of which occur in this work on the tautomerism process in enhancing the proton transfer from the O-H to N-H i.e towards the formation of the keto tautomer.
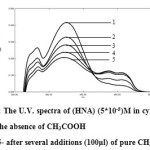 |
Figure 3: The U.V. spectra of (HNA) (5*10-5)M in cylohexane. Click here to View figure |
1- in the absence of CH3COOH
2,3,4,5- after several additions (100μl) of pure CH3COOH
Finally it can be concluded that the lanthanide shift reagent can act as a tautomeric agent for orthohydroxynaphthylidene aniline supported by the above results.
References
- S. Bilage, Z.Kilic, Z.Hayvali, T.Hokelek and S.Safran, J. Cham. Sci., Vol.121, 6, 989-1001 (2009).
- O. Dominguez, B. Rodriguez-Molina, M.Rodriguez, A. Afriza, N. Farfan and R. Santillan, New J. Chem., 35, 156-164 (2011).
- A. Blagus, D. Cincic, T. Friscic, B. Kaitner and V. Stilinovic, Macdonian J. Chem. Chemical Engineering, 29, 2, 117-138 (2010).
- A.M. Asiri, K.O. Badahdah, S.A. Khan, A. G. Al-Sehemi, M.S. Al-Amoudi and A.A. Bukhari, organic chemistry Insights, 3,1-8 (2010).
- D. Nedeletcheva, F.S. Kamounahm, L. Mirolo, K.M. Fromm and, L.Antonov, Dyes and pigments, 83, 1; 121-126 (2009).
- J. Matijevic-Sosa, M. Uinkovic, and D.V. Topic, Croatica chemical Acta, 79, 3, 489-495 (2006).
- P.K. Malik, ph. D. thesis, Dept. of Chemistry, National Institute of Technology, (2010).
- P.E. BOGDAN, ph.D. thesis, L’Universite’ Bordeaux1, 6 mai (2011).
- W.M.F. Fabian, L. Antonov, D. Nedeltcheva, F. Kamounah, and P.J. Taylor, J. Phys. Chem. A, 108, 7603-7612 (2004).
- M. Khuba, P. Lipkowski A. Filarowski, Chem. Phys. Letters, 463, 1, 426-430 (2008).
- W.M.F. Fabian, L. Antonov, D. Nedel tcheva, and F.S. Kamounah, Institute fur chemiem, Karl-Franzens, UNI, GRAZ, (2011).
- L.M.N. Saleem, Organic magnetic Resonance, 19, 176 (1982).
- L.M.N. Saleem ,A. T. AL-Razzak, Raf.J.Sci., 11, 84 (2000).
- J.M.AL-Rawi, L.M.N.Saleem, Magn. Res. Chemi, 27, 540 (1998).
- N. Tezer and N. Karakus, J. Mol. Modeling, 15, 3, 223-232 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









