Comparative Surface Study of Corrosion Behavior of Carbon Steel with Nickel, Copper and 70/30 Ni-Cu Alloy in Seawater-Part 1
A. Hamdy*, Khaled Z. Mohammed, Mohamed A. Abbas and Al-Sayed M. Aly
Egyptian Petroleum Research Institute (EPRI), Nasr City, Cairo, Egypt.
Comparison of corrosion behavior of Ni, Cu and 70/30 Ni-Cu alloy together with that of carbon steel in seawater has been investigated. Characteristics such as morphological changes and composition of corrosion products have been studied comparatively by means of scanning electron microscopy (SEM), energy dispersive X-ray (EDX) and X-Ray diffraction (XRD). It was found that both the protective properties and adherence of the film, formed on the surface depend on the nature of the substrate and the environment composition. Corrosion mechanisms for all the studied materials were suggested based on the surface analyses. The results show that the chloride content is the controlling factor in corrosion of both carbon steel and copper in seawater, while sulphur content is the regulating factor for corrosion of nickel and 70/30 Ni-Cu alloy in seawater.
KEYWORDS:Corrosion; Carbon steel; Sea water
Download this article as:| Copy the following to cite this article: Hamdy A, Mohammed K. Z, Abbas M. A, Aly A. S. M. Comparative Surface Study of Corrosion Behavior of Carbon Steel with Nickel, Copper and 70/30 Ni-Cu Alloy in Seawater-Part 1. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Hamdy A, Mohammed K. Z, Abbas M. A, Aly A. S. M. Comparative Surface Study of Corrosion Behavior of Carbon Steel with Nickel, Copper and 70/30 Ni-Cu Alloy in Seawater-Part 1. Available from: http://www.orientjchem.org/?p=11887 |
Introduction
Seawater is one of the most abundant naturally occurring electrolytes, covering about 70% of the earth’s surface. Seawater systems are used by many industries such as shipping, offshore oil and gas production, power plants and coastal industrial plants. The main use of seawater is for cooling purposes but it is also used for fire-fighting, oil field water injection and for desalination plants.1,2 Seawater is inherently chemically aggressive, and therefore, constructional materials used in seawater handling and processing systems including desalination plants are subjected to varying degree of corrosion depending upon the nature of the materials and operational conditions. Broadly speaking, for applications in seawater, the materials are usually exposed to three zones, namely, fully immersed, partially immersed or seawater surrounding environment.3
Marine corrosion includes the immersion of components in seawater, equipment and piping that use seawater or brackish water, and corrosion in marine atmospheres. Exposure of components can be continuous or intermittent. Ships, marinas, pipelines, offshore structures, desalination plants, and heat exchangers are some examples of systems that experience marine corrosion. The corrosion problems in these systems have been well studied over many years; despite several published information on materials behavior in seawater,4,5 failures still occur. Therefore, more investigations need to carry out to obtain better understanding on material corrosion behavior.
Seawater is a complex mixture of inorganic salts (mainly sodium chloride), dissolved gases (notably oxygen), suspended solids, organic matter and organisms.6 Singh et al.7 founded that the presence of salinity and SO2 in the environments changed the corrosion characteristics, structure and protective properties of rust formed on the surfaces of steels. Chloride ion is one of the most significant natural contaminant in marine environment which plays a big role in the corrosion process of structural steel.8 Sulfide in seawater can occur in many ways, such as from rotting vegetation, from industrial waste discharge and from the biological and bacteriological process in seawater (e.g. the naturally occurring sulfate in seawater reduced by sulfate-reducing bacteria).9
Copper and its alloys are very widely used materials in many industrial applications because of their excellent electrical and thermal conductivity and their corrosion resistance.10-12 There are a lot of pipes, tanks, valves, tubes in condensers and heat exchangers are made from copper in the industries. Nickel is an important metal and it is used in a large number of industrial applications.13 Nickel is widely used as an alloying element because of its toughness and corrosion resistance.14 However, copper-nickels have been specified for sea water use for over 50 years; they are the materials of first choice for seawater pipe work and condenser service for many of marine applications. They are used in desalination, power plants and offshore fire water systems, and for the sheathed protection of oil and gas platform legs and risers.15,16
Carbon steel is used in mass amounts in marine applications, chemical processing, petroleum production and refining, construction and metal-processing equipment17,18; despite it has a relatively high cost. These applications usually induce serious corrosive effect on equipments, tubes and pipelines made of iron and its alloys.19
The above considerations prompted us to study characteristics such as morphology and composition of corrosion layers formed on the surface of the different studied metals and alloys by means of SEM, EDX and XRD to throw light on the corrosion mechanisms of these studied materials in sea water. This work will be continued in another part by studying the corrosion behavior of the studied materials in desalinated seawater.
Experimental
Materials
The materials selected for this study were carbon steel alloy (CS), copper (99.1%purity), nickel (99.9% purity) and nickel-copper alloy (Monel_400). The CS coupons have percent composition (wt.%) of 98.38% Fe, 0.28% C, 1.25% Mn, 0.05% S and 0.04% P, while the percent composition of used Monel_400 is 62.4 Ni, 34.7 Cu, Fe 0.7 and Mn 1.2 . The compositions of the specimens were analyzed by Portable X-ray Fluorescence (XRF) model NITON_XLt driven with software version 4.1.All the tested materials were provided by Center Metallurgical Research and Development Institute (CMRDI) and cut into coupons with 4.0 cm x 2.0 cm x 0.15 cm dimensions.
Prior to all surface analyses,the different tested coupons were immersed in sea water for different time intervals .Before immersion all the test specimens were surface – ground manually on successively finer silicon carbide emery papers with (350, 1000, and 1200) grit finish on all faces, degreased with acetone, rinsed in tap and distilled water respectively, and finally dried in warm air.
Solution
The tested solution is stagnant, naturally seawater obtained from Mediterranean Sea. Characteristic properties of seawater samples including pH, density, specific gravity, electrical conductivity, resistivity, total dissolved solids, salinity, and hardness were determined according to the standard methods,20-24 and the results are given in table1. Cationic and anionic analyses were performed on the used seawater by ion chromatography, using DX 600 gradient IC system (Dionex, Sunnyvale, CA, USA). Integration was performed by Chromeleon Ver. 6.30 software (Dionex), and the results are listed in table 2.
Table 1: Physico-chemical properties of seawater
|
38500mg/l |
Total dissolved solids |
|
5.6 x10-2 ohms/cm @23.7 oC |
Electrical conductivity |
|
0.17857 ohm-m @ 23.7oC |
Resistivity |
|
35036 mg/l |
Salinity |
|
7.606 @25oC |
pH |
|
1.03036 g/ml @60 oF |
Density |
|
1.03139 |
Specific gravity |
|
7535 mg/l |
Hardness |
Table 2: Cationic and anionic analysis of sea water.
|
Concentration mg/l |
Constituent |
|
11583.3 |
Na+ |
|
387.35 |
K+ |
|
1433.8 |
Mg2+ |
|
652.96 |
Ca2+ |
|
21234 |
Cl– |
|
3009.2 |
S2– |
Scanning Electron Microscopy
Specimens of different studied materials, after exposing to seawater for 30 days, were observed by mean of the Scanning Electron Microscopy. The test was performed using JEOL-model JSM-53000 scanning electron microscope (SEM). The working sample was analyzed at three different locations to ensure reproducibility.
Energy Dispersive Spectroscopy
The composition of the surface film formed on the metal specimens was examined by energy dispersive X-ray (EDX (. This was carried out with X-Max Oxford energy dispersive spectrometer conjugated with transmission electron microscope Jeol 2100. The spectra were recorded on samples immersed for a period of 48 hrs in natural seawater.
X-Ray Diffraction
The corrosion products developed on the surface of the studied coupons, as a result of 30 days immersion in sea water, were taken up, gently powdered and homogenized, then examined using X-ray powder diffractometer, Panalytical XPERT PRO MPD. Cu Kα radiation ( λ= 1.5418 Aο) was used at a rating of 40 kV, 40 mA. The diffraction patterns were recorded at room temperature in the angular range of 4ο-80ο (2θ) with step size 0.02o (2θ) and scan step time 0.4 (s). The crystalline phases formed on the carbon steel surface, in both cases, were identified using the ICDD-PDF database.
Results and Discussion
SEM analysis
Examination of the corroded surfaces of different studied materials by SEM is shown in Fig. 1. The micrograph of corroded carbon steel surface (Fig.1a) shows a homogeneous corroded area, with spots of localized attack. This condition is observed throughout the surface sample .Also, a number of small and shallow pits were formed on the carbon steel surface as a result of exposure to sea water. Fig.1 b shows the changes in surface morphology of copper specimen after immersion in the corrosive tested media. Small pits are observed on the copper surface due to the attack of chloride ions and dissolved oxygen as; both contribute to the oxidation of the metal.25 In case of nickel metal (Fig.1 c) although the surface of the specimen still somewhat bright but localized corrosion in the form of a fine dark spots appear among the scratches. The micrograph of Fig.1 d shows that the surface of Ni-Cu alloy has some roughness and characterized in some locations by the occurrence of some pits and suffering from some cracks. The micrograph indicates that, the alloy suffer from general as well as pitting corrosion.
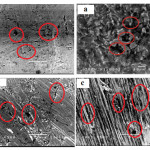 |
Figure 1: Micrographs of different studied materials after immersion in sea water for 30 days. Click here to View figure |
EDX analysis
In order to define more accurately the nature of the corrosion layers observed in each condition elemental mappings were performed by EDX. Fig.2 presents an EDX panorama recorded for different studied samples after exposure for 48 hrs in seawater. The elemental composition of the corrosion products formed on the surfaces of different studied materials is reported in table 3. Both, the EDX spectra of the film formed on carbon steel surface (Fig. 2a) and the data reported in table 3 confirm the existence of iron oxide layer as indicated from the clear existence of Fe and O signals. Also, the characteristics peaks of some of the elements constituting the carbon steel sample are shown in the spectra like C and Si .In the case of copper metal the EDX spectra shown in Fig. 2b indicates that Cu, Cl and O are the major constituents of the film formed on copper surface as indicated from their high intensity signals and from the data listed in table 3. This leads us to the suggestion that, a complex layer is formed on copper surface as will be discussed later. The spectra of the corrosion layer formed on both nickel and nickel -copper surfaces is shown in Fig. 2c and d respectively. The EDX spectra and the data of table 3 show that, the main elements which were present on the nickel surface are Ni and S, while in the case of Ni-Cu alloy the highest signals correspond to Ni, Cu and S signals. These data show that sulfur has high contribution in the composition of the corrosion products formed on Cu and Ni-Cu surfaces.
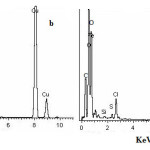 |
Figure 2: EDX spectra of the film formed on the surfaces of the studied materials after immersion in seawater for 48 hrs. Click here to View figure |
(%Table 3: EDX results of corresponding materials (mass fraction
|
Element |
Carbon steel |
Copper |
Nickel |
Nickel-Copper |
|
Fe |
62.66 |
– |
– |
– |
|
C |
7.68 |
– |
– |
– |
|
Cu |
– |
64.29 |
– |
50.14 |
|
Ni |
– |
– |
67.67 |
16.22 |
|
O |
26.94 |
18.18 |
5.23 |
4.23 |
|
S |
0.4 |
1.2 |
26.14 |
28.73 |
|
Cl |
2.32 |
16.33 |
0.96 |
0.68 |
|
Total |
100 |
100 |
100 |
100 |
XRD analysis
The diffraction pattern of carbon steel after 30 days of immersion shows the presence of small amounts of tetragonal iron oxide hydroxide (Lepidocrocite) phase, card no. (060-0344), where its characteristics peaks located at 2θ :14.19 and 26.96. The detected FeOOH phase on C-Steel surface leads us to the suggestion that, initiation of corrosion results in the formation of this oxide layer. However, this layer is not protective against corrosion and part of this oxide layer is readily cracks allowing the ingress of seawater to the metal surface causing further corrosion26 This explains the presence of multiphase iron oxide layer where, the most dispersed peaks of the diffraction pattern at values of 2θ : 30.15, 35.55 ,43.19, 57.14 and 62.63 are characteristic of Fe3O4 (Magnetite) and Fe2O3 (Hematite) with cubic and tetragonal crystal structure , respectively cards no. (001 – 1111) and (002 – 0915). The formation of these oxides can be attributed to that on further immersion, a part of the previously formed oxide transforms to protective magnetic oxides of iron composed of Fe3O4 and Fe2O3 double layer.27
Diffraction pattern characterized the corrosion products formed on copper surfaces in seawater is represented in Fig.3b. It shows the characteristic peaks of copper chloride hydroxide complex [Cu46 Cl24 (OH) 68] card no. (023-0950), which are identified at (2θ = 16.27, 31.78, 39.87 and 45.55)
The diffraction pattern of the corrosion products formed on Ni surface upon exposure to seawater (Fig. 3c,) shows the phases of NiS (Millerite) card no. (04-06 -5315) .The peaks were detected at (2θ:29.31, 31.6, 43.09 ,45.37 and 48.9).The diffraction pattern belongs to the corrosion products formed on Ni-Cu surface as a result of exposure to sea water( Fig.3d) reveals the presence of copper nickel sulfide phase Cu0.1Ni0.9S2 card no. (04-006- 4466) at positions (2θ = 45.45and 56.55), also the characteristic peaks of NiS2 card no. (011-0099) are detected at (2θ :27.29 and 31.65 ).
 |
Figure 3: Diffraction Patterns for the corrosion products formed on the surfaces of studied materials, after exposure to seawater for 30 days.
|
Corrosion Mechanisms
Carbon steel alloy
The results obtained from the different surface analyses indicate that the main corrosion products formed are Fe3O4 andγ – F2O3. This, lead to the suggested corrosion mechanism of carbon steel in sea water based on the following facts, the reduction of water forms the cathodic reaction in the overall corrosion reaction as follows:
2 H2O + 2e ‾ = H2 + 2 OH– (1)
This gives rise to the formation of iron II species, Fe2+, which changes to iron II hydroxide,
Fe(OH)2, and then transform to magnetite, Fe3O4 [28]. The overall reaction is represented by:
3 Fe + 4 H2O = Fe3O4 + 4 H2 (2)
This reaction proceeds in stages [29]:
Fe + H2O = Fe. H2O (ad.) (3)
Then, this reaction is followed by one of two reaction steps as follows:
Fe. H2O (ad.) = Fe OH (ad) + H+ + e– (4)
or
Fe. H2O (ad) = Fe OH– (ad) + H+ (5)
and
Fe OH– (ad) = Fe OH (ad) + e– (6)
Fe OH (ad) + OH– (aq) = Fe (OH) 2 + e– (7)
3 Fe (OH) 2 = F3O4 + 2 H2O + H2 (8)
According to the all above facts, we can say that when carbon steel is immersed in seawater for a long period of time, the metal will be rapidly dissolved and involved in forming the first corrosion product Fe (OH)2 as indicated in equation (7), this product is responsible for the increase in corrosion rate. Also, one possible reason for the increase in corrosion with time can be ascribed to the initial formation of pits on the steel surface. As the transition time is achieved, the composition of the corrosion product layer changes from Fe (OH)2 to predominantly Fe3O4 protective film as shown in equation (8). Then, Fe3O4 is partially transformed to γ – Fe2O3 as given in equation (9) which corresponds to the formation of stable passivity layer.
Fe3O4 + H2O = γ – F2O3 + 2 H+ + 2 e– (9)
Some authors,30,31 assumed that the passive film is consisted of Fe3O4 layer facing the metal, and γ – Fe2O3 facing the electrolyte. This film retards the corrosion process as it can limit or arrest further metal dissolution by acting as a physical barrier to the corrosion reaction, resulting in a decrease in corrosion rate .Also, the rate of corrosion is controlled by diffusion through the corrosion product layers, i.e., when the thickness of the oxide layer increases the diffusion rate decreases resulting in simultaneous decrease in corrosion rate with time.32 However, it is expected that, on further increase in immersion time, a sudden increase in corrosion rate is taking place due to breakdown of passive film by the action of chloride ions, due to their breakdown action on the passive film.33 The chloride ions may be associated with the ability of these anions to adsorb on the passive film (at the bottom of cracks and pores in the oxide film) causing increase in corrosion rate.34 As natural seawater (the test media) is rich in chlorides, so breakdown of protective layer is expected. So, it can be said that chloride ion is the regulating factor in corrosion mechanism of carbon steel in sea water.
Copper
It is generally accepted that copper dissolution is influenced by chloride concentration [35] .This assumption is in agreement with the results of all the surface analyses of the film formed on Cu surface upon exposure to seawater, as copper chloride complex was the main phase of the formed corrosion products. Due to the activation effect of chloride ions, copper dissolution can proceed according to one of these following proposed mechanisms [36]:
(I) Cu +2 Cl – = CuCl2– + e– (10)
(II) Cu = Cu+ + e– (11)
( Cu+ + 2Cl– = CuCl2 (12)
(III) Cu+ Cl– = CuCl + e– (13)
CuCl+Cl– = CuCl2- (14)
CuCl2- is believed to control the kinetics of anodic dissolution of copper in chloride solutions. It was reported that the rate of copper dissolution depends on diffusion of the soluble forms of CuCl2- into the bulk of solution.37 However, the method of cuprous oxide production in the presence of the chloride ion is usually taken as a precipitation reaction rather than a direct electrochemical or chemical formation from the base metal or cuprous chloride.38 The equilibrium in reaction (15) is shifted to the right as the local concentration of CuCl2- complex (produced directly from the dissolution of copper metal or CuCl) increases and cuprous oxide is deposited in response.
2CuCl2- +2OH – ↔ Cu2O + H2O +4Cl – (15)
The stability of Cu2O is inversely dependent on the concentration of chloride ions. Thus, from the general literature dealing with copper corrosion, the rate of re-dissolution of the protective Cu2O (as a soluble cuprous chloride complex) has been shown to be much higher than that observed in chloride free solution [39]. Hence, it can be deduced that the increase in chloride ion concentration leads to the attack on the insoluble CuCl layer, which transforms into soluble CuCl2- complex and also, enhances the dissolution of Cu2O layer and hence higher corrosion rate is observed in the rich chloride medium.
Nickel
The main phase of the corrosion products formed as a result of corrosion of nickel in sea water was nickel sulphide .This result can be attributed to the fact that, pure nickel is more susceptible to corrosion in sulfide-polluted solutions.40 This was previously interpreted by the conclusions of Marcus P. et al,41 who attributed the high corrosion rate of nickel in sulphur-containing solutions to the formation of a porous three-dimensional nickel-sulphide layer formed on the electrode surface. It was suggested that, in the first stage of the corrosion process a thin adherent film of nickel sulfide bonded to the metal surface tightly, and hence hinder the dissolution of nickel resulted, in a decrease in corrosion rate,42 but on further increase in time an increase in corrosion rate is observed which may be due to the damage of the sulfide layers formed in the early stage and the replacement of those layers with another sulfide layers, in which the bonds of the latter layers with the metal surface were bad, hence their dissolution-hindering effect was not good. This caused the increase of nickel dissolution and consequently the rise in corrosion rate.
Ni-Cu alloy
Previous studies43,44 confirmed that the co-existence of sulfide and oxygen highly accelerated the corrosion rate of nickel and nickel alloys. This fact explains the results of surface analyses which indicate the presence of copper nickel sulphide and nickel sulphide as corrosion products for Ni-Cu corrosion in seawater. It is accepted that the corrosion rate of Ni-Cu alloy was linked to the sulfide concentration,45 the rapid failure of Monel_400 alloy in a sulfide-containing seawater may be attributed to the hypothesis that the porous, non-protective film formed of copper and nickel sulfide inhibited the formation of a protective oxide film.46
This behavior can be explained as follows: at the pH of seawater, the sulfide ion is not stable and hydrosulfide is dominant:
S2- (aq.) + H2O → HS– (aq.) + OH– (aq.) (16)
Then, the following reactions proceed:
NiCu (s) + HS– (aq.) →NiCu(HS–)ads → Cu(HS–) ads +Ni(HS–) ads (17)
Cu (HS–) ads→ Cu (HS–) (s) + e– (18)
Ni (HS–) ads →Ni (HS) + (aq.) + 2e– (19)
Cu (HS-) (s) → Cu+(aq.) + HS– (aq.) (20)
Ni (HS) + (aq.) → Ni2+ (aq.) + HS– (aq.) (21)
Then, the reaction of Cu+ and Ni2+ ions with HS– can produce cuprous sulfide (Cu2S) and nickel sulfide (NiS) as follows:
2 Cu+ (aq.) + HS– (aq.) + OH– (aq.) → Cu2S (s) + H2O (l) (22)
Ni2+ (aq.) + HS– (aq.) + OH– (aq.) → NiS(s) + H2O (l) (23)
Therefore, the corrosion product layer on the alloy is a porous, non-adherent and non protective mixture of copper and nickel sulfides. This layer adsorbs on alloy surface and provides partial protection , but on further increase in time of immersion this weak layer splits from the surface and hence, a rapid increase in corrosion is observed .
Conclusions
- The metallographic SEM examinations of the studied materials indicate the occurrence of general corrosion of the studied metals and alloys. However, pitting as well as intergranular corrosion was also observed due to the sulphur content in the tested media.
- The EDX and XRD results show that, upon exposure of the studied materials to seawater, the major phases of corrosion products formed on carbon steel is Fe –oxide phases of both Fe3O4 and Fe2O3,while copper chloride hydroxide complex phase is formed on copper surface . The corrosion products detected on nickel surface is nickel sulfide, while those formed on Cu-Ni alloy are copper nickel sulfide phase along with nickel sulfide.
- Suggested corrosion mechanisms for the studied materials in seawater reveal that the chloride content is the controlling factor in corrosion of both carbon steel and copper, while sulphur content is the regulating factor for corrosion of nickel and 70/30 Ni-Cu alloy in seawater.
References
- Philip L., J. Marine Technology, 27,135( 1990).
- Dreizin Y., Desalination, 190, 104 (2006).
- Saleh A. Al-Fozan, Anees U. Malik, Desalination, 228,61 (2008).
- Bethencourt M., Botana F.J., Cauqui M.A., Marcos M.and Rodriguez M.A., J. Alloy Compd. 250, 455 (1997).
- Wharton J.A., Barik R.C., Kear G., Wood R.J.K., Stokes K.R.and Walsh F.C., Corros. Sci. 47, 3336 (2005).
- La Que F. L., Marine corrosion causes and prevention, John Wiley &sons, New York 116 (1975).
- Singh D.D.N., Yadav S., Saha J.K., Corros. Sci., 50 , 93(2008).
- Yuantai Ma, Ying Li, Fuhui Wang, Corros.Sci., 51,997 (2009) .
- Yuan S.J.and Pehkonen S.O., Corros. Sci. ,49,1276 (2007) .
- Barouni K., Bazzi L., Salghi R., Mihit M., Hammouti B., Albourine A. and El Issami S., Mater. Lett., 62, 3325 (2008) .
- Musa A. Y., Mohamad A. B., Kadhum A. A. H. and Tabal Y. B. A., J. Mater. Eng. Perf., 20 , 394 (2011) .
- Musa A. Y., Kadhum A. A. H., Mohamad A. B. and Takriff M. S., Inter. J. Surf. Sci. Eng.,5, 226 (2011).
- Hamed E., Abd El-REhim S.S., El-Shahat M.F., Shaltot A.M., Mater. Sci. and Eng. , 177, 441 (2012) .
- Kear G., Barker B.D., Stockes K.R., Walsh F.C., J. Appl. Electrochem., 34, 1235 (2004).
- Hamed E., Abd El-REhim S.S., El-Shahat M.F., Shaltot A.M., Materials Science and Engineering B, 177, 441 (2012).
- G.Y. Elewady, A.H. El-Askalany, M.F. Molouk, Port. Electrochim Acta ,26,503 (2008).
- Zhang S.T., Tao Z.H., Li W.H., Hou B.R., Appl. Surf. Sci., 255 , 6757 (2009).
- Lashgari M., Arshadi M.R., Miandari S., Electrochim. Acta 55, 6058 (2010).
- Ayse Ongun Yuce, Gulfeza Kardas, Corros. Sci., 58 , 86 (2012).
- Standard Test Method ASTM D 1293 for pH of Water, Philadelphia, Vol.11.01, 274 (1992).
- Standard Test Method ASTM D 1429 for Specific Gravity of Water and Brine, Vol. 11.01, Philadelphia, 301 (1992).
- ” Standard Test Method ASTM D 1125 for Electrical Conductivity and Resistivity of Water”, Vol. 11.01, Philadelphia, 202 (1992).
- Eaton A.D., Clesceri L.S. and Greenberg A.E, Standard Methods for the Examination of Water and Waste Water, 19th Edn., Washington, (1995).
- Standard Test Method ASTM D 3875 for Alkalinity in Brackish Water, Seawater and Brines, Vol. 11.02, Philadelphia , 437 (1992).
- Scendo M., Corros.Sci., 50, 2070 (2008).
- T. Misawa, T. Kyuno, W. Suetaka and S. Shimodaira, Corr. Sci, Vol. 11, 35, (1971).
- C. Wagner and B. Bunsenges, Phys. Chem., Vol 77, 1090, (1973).
- Robertson J., Corros. Sci, 29, 1275 (1989).
- Ashley G. W. and Burstein G. T., Corrosion, 47, 908 (1991).
- Vetter K. J., Electrochemical Kinetics, Theoretical and Experimental Aspects, Academic Press, New York, (1967).
- Philipe Marcus, Corrosion Mechanisms in Theory and Practice, Marcel Dekker, (2002).
- Al – Hajji J. N. and Reda M. R., Corrosion, 49, 363 (1993).
- Janik – Czachor M., J. Electrochem. Soc., 129, 513 (1981).
- Murphy O. J., Pou T. E., Young E. V., Bockris J. O’ M and Tongson L. L., J. Electrochem. Soc., 131, 1243 (1984).
- Wood, R. J. K., Hutton, S.P., Schiffrin, D.J., Corros. Sci., 30,1177 (1990).
- Zhang, D. Q., Gao, L. X., Zhou, G. D., Appl. Surf. Sci., 225 ,287 (2004).
- Milan M. A., Snezˇana M. M. and Marija B. Petrovic., Corros. Sci., 51 ,1228 (2009) .
- Faita, G., Fiori, G. and Salvadore, D., Corr. Sci.,15 , 383 (1975).
- Vazquez, M. V., de Sanchez, S. R., Calvo, E. J.and Schiffrin, D. J., J. Electroanal.Chem.,374 ,179 (1994).
- Abdallah, M., Al karanee, S. O. and Abdel Fattah, A. A., ZaŠtita Materijala., 50 ,205 (2009).
- Marcus, P., Teissier, A., Oudar, J., Corros. Sci., 24 ,259 (1984).
- Xiaoliang Cheng., Houyi Ma., Shenhao Chen., Xiao Chen., Zhiming Yao., Corros. Sci., 42 ,299 (2000).
- Mansfeld F., Liu G., Xiao H., Tsai C. H., Little B. J., Corr. Sci., 36, 2063 (1994) .
- Al-Hajji, J. N. and Reda, M.R., Corros. Sci., 34, 163 (1993).
- Beccaria, A. M., Poggi, G., Traverso, P. T.and Ghiazza, M., Corros. Sci., 32 ,1263 (1991).
- Elselstein, L. E., Sytett, B.C., Wing, S. S. and Caligiuri, R. D., Corros. Sci., 23,223 (1983).

This work is licensed under a Creative Commons Attribution 4.0 International License.









