The Si-doped Zigzag (6,0) AlN Nanotube: A Computational NMR Study
Reza Soleymani1 *, Farzad Torkashvand2, Sahar Farsi-Madan3 and Mohammad Bayat2
1Young Researchers Club, Shahre-rey Branch, Islamic Azad University, Tehran (Iran). 2Department of Chemistry, Toyserkan Branch, Islamic Azad University, Toyserkan (Iran). 3Payame Noor University (PNU), Shiraz (Iran).
By using of density functional theory (DFT) in theory level of B3LYP and 6-31G(d) basis set, zigzag (6,0) aluminum nitride nanotubes (AlNNTs) structures was investigated in perfect and Si-dope states and for this purpose, NMR parameters involving Chemical Shift Isotropic and Chemical Shift Anisotropic by using GIAO method were investigated. Obtained results were shown that by doping Silicon nucleus on AlN nanotube structure in perfect state, values of NMR parameters were varied for different nucleuses, these variation were depend on position of each nucleus in nanotube structure and chemical medium of nucleus based of neighbor nucleus. However, Si-dope on nanotube structure was caused to changing of regular process for bond angle and bond lengths that existed in perfect state.
KEYWORDS:NMR; DFT; AIN Nanotube; Si-dope
Download this article as:| Copy the following to cite this article: Soleymani R, Torkashvand F, Farsi-Madan S, Bayat M. The Si-doped Zigzag (6,0) AlN Nanotube: A Computational NMR Study. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Soleymani R, Torkashvand F, Farsi-Madan S, Bayat M. The Si-doped Zigzag (6,0) AlN Nanotube: A Computational NMR Study. Available from: http://www.orientjchem.org/?p=11850 |
Introduction
Silicon elements are in IV groups in periodic table that are similar to properties of Carbon and Germanium elements. So, these compounds can be used instead of Carbon and Germanium elements [1]. But investigating of properties of these compounds as doping nucleus in nanotubes instead of some elements like Carbon and Germanium or III and IV groups in periodic table is investigated [2,9]. Till 1980, three carbon allotrope (none-crystallization carbon) named Diamond, Graphite and Amorphous carbon were discovered, first carbon allotrope that discovered in 1985, was Buckminsterfullerene that however named Buckyball and fullerene [10]. Fullerenes are spherical molecules of carbon that are interesting because of their wonderful properties and beautiful shape [10]. In 1991, other carbon structure was discovered by Sumio Iijima accidently that had unique properties [11]. Iijimia worked in a laboratory in NEC Company in Japan as a electronic microscope expert. When he used transition electronic microscope for observation of fumy products, accidently, observed carbon nanotubes that attached to carbon graphite cathode [1]. At first, he imaged it as a fullerene that drew in a direction. But finally he noticed that this structure was different from fullerene and because of it, it was named carbon nanotube. In carbon nanotubes, carbon atoms are arranged in cylindrical structures [11]. It means that it`s carbonic. Nanotubes are annular compound that in general are single-wall and multi-wall. Van der Waals force is made between multi-wall nanotube. However single-wall models of these compounds in various models are in zigzag, chiral and armchair models. These more applicable nanotubes are involving C-C nanotubes, B-N nanotubes and Al-N nanotubes [2,9]. Al-N nanotubes are more applicable that is because of different physical, chemical properties and electrostatic forces between Al and N nucleuses. In these last years, many studies are done about this compound theoretically and experimentally [13,14]. In fact, researchers want to find new way that by changing of structural properties of these compounds, electrical conductivity, thermal conductivity and application of this compound as Nano-Drug Carriers (NDCs) and as compounds with unique chemical properties can be changed. So, investigating of NMR values parameters can be used in investigating of these compounds. Investigating of NMR parameter gives some information about chemical shift of various nucleus and chemical field around of nucleuses. In fact, investigation of these parameters is done by investigating of chemical shift isotropic (CSI) and chemical shift anisotropic (CSA). In 2008, studies were done about Al-N nanotube by Mirzaei et al and investigated doping effects of Carbon nucleus on structure of these compounds [13]. However in 2009, similar studies were done by Farahani et al about these compounds, different states of Al-N nanotubes and B-N nanotubes were compared [14]. But in this study, in a new way, effect of Silicon group are investigated on NMR parameter of Al-N nanotube structure in zigzag (6,0) state.
Computational Methods
By using density functional theory (DFT) in theory level of B3LYP and 6-31G(d) basis set, structure of Al-N nanotube with 10 Angstrom length in zigzag (6,0) model, Si-dope and perfect states was investigated. At first, Al-N nanotube structure was designed in perfect and Si-dope states and nuclear magnetic resonance (NMR) parameter values for these structures were investigated. For this purpose, Chemical Shift Isotropic (CSI) and Chemical Shift Anisotropic (CSA) values of these structures were calculated and quality of these changing was considered [12-14]. That following equations were used for investigating of these parameters. Chemical shift tensors in the principal axes system (PAS) (σ33 > σ22 > σ11) calculated by mechanic quantum calculation.
(CSI (ppm) = (σ11 + σ22 + σ33)/3 (1)
(CSA (ppm) = σ33 − (σ11 + σ22)/2 (2)
All calculation was done in Gas phase, 1atm pressure and 298 K temperature and used Gaussian 09w program of package [15]. In general, all calculations were done by Pentium IV PC by intel® processor 1.73 GHz, core i7 and 4 GB of memory in XP® operating system. At first Al-N nanotube structure in zigzag (6,0) model was optimized by AM1 method and finally optimized by DFT method. Molecular formula of Al-N nanotube is Al24H12N24 in perfect state and is Al21 H12N21Si6 in Silicon dope state. In this state, 6 Silicon atoms are doped on Al-N nanotube structure.
Discussion and Results
At first structures of Al-N nanotube in zigzag (6,0) model in perfect (fig 1) and Si-dope states (fig 2) are optimized by theoretical methods. All obtained results are reported in table 1 to 3, involving results of structural parameter, CSA and CSI values in different states of AlN nanotube.
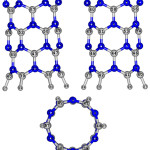 |
Figure 1: 2D views of the perfect (6,0) AlNNTs.
|
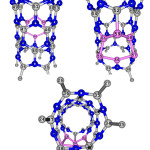 |
Figure 2: 2D views of the 14Si-dope (6,0) AlNNTs.
|
Geometrical parameter
Bond length
Obtained results of bond length and bond angles are reported in table 1 and 2 respectively. All results are shown that calculated values are changed by addition of Silicon nucleus to AlN nanotube. Investigating of values bond length is shown that it is 1.019 angstrom for N-H in perfect state and variation range is 1.018 to 1.021 Å in Si-dope state. But bond length value in Al-H is 1.582 Å in perfect state and it`s varied between 1.577 to 1.586 Å in Si-dope state. Investigating of bond length of Al-N is shown that it`s not varied in perfect state and its variation range is between 1.807 to 1.818 Å. However, in Si-dope state, variation range is increased and it`s because of addition of Silicon nucleus. Minimum amount is 1.807 Å in perfect state and maximum amount is 1.818 Å that is smaller in similar bonds. Minimum amount for bond length of Al-N in N11-Al53 for Si-dope state is 1.800 Å and maximum amount is in N8-Al45 and its 1.860 Å. But other bond length are in range of 2.414 to 2.386 Å that is because of increasing of atomic radius. Variation range of Si-Si is between 2.303 to 2.386 Å.
Table 1: Bond length (Angstrom) in perfect and Si-dope (6,0) zigzag AlNNTs.
|
Zigzag (6,0) AlNNTs Si-Dope |
Zigzag (6,0) AlNNTs Perfect |
||||||
|
Bond length |
Bond length |
||||||
|
N1- H30 |
1.019 |
N14- H33 |
1.018 |
N1- H33 |
1.019 |
N14- Al49 |
1.816 |
|
N1- Al49 |
1.808 |
N14- Al51 |
1.819 |
N1- Al55 |
1.815 |
N14- Al50 |
1.816 |
|
N1- Al50 |
1.815 |
N14- Al53 |
1.814 |
N1- Al56 |
1.815 |
N14- Al59 |
1.807 |
|
N2- Al48 |
1.866 |
N15- Al46 |
1.813 |
N2- Al53 |
1.816 |
N15- Al47 |
1.815 |
|
N2- Al50 |
1.850 |
N15- Al47 |
1.813 |
N2- Al54 |
1.816 |
N15- Al48 |
1.815 |
|
N2- Si58 |
1.743 |
N15- Al51 |
1.804 |
N2- Al56 |
1.807 |
N15- Al49 |
1.811 |
|
N3- Al41 |
1.824 |
N16- Al40 |
1.813 |
N3- Al45 |
1.815 |
N16- Al37 |
1.818 |
|
N3- Al42 |
1.816 |
N16- Al43 |
1.813 |
N3- Al46 |
1.815 |
N16- Al38 |
1.818 |
|
N3- Al48 |
1.809 |
N16- Al46 |
1.802 |
N3- Al54 |
1.811 |
N16- Al47 |
1.814 |
|
N4- Al37 |
1.804 |
N17- Al35 |
1.818 |
N4- Al40 |
1.818 |
N17- H36 |
1.019 |
|
N4- Al38 |
1.811 |
N17- Al36 |
1.816 |
N4- Al41 |
1.818 |
N17- Al57 |
1.815 |
|
N4- Al42 |
1.823 |
N17- Al40 |
1.809 |
N4- Al46 |
1.814 |
N17- Al59 |
1.815 |
|
N5- H28 |
1.021 |
N18- H31 |
1.018 |
N5- H31 |
1.019 |
N18- Al50 |
1.816 |
|
N5- Al50 |
1.806 |
N18- Al49 |
1.814 |
N5- Al56 |
1.815 |
N18- Al52 |
1.816 |
|
N5- Al52 |
1.817 |
N18- Al51 |
1.818 |
N5- Al58 |
1.815 |
N18- Al57 |
1.807 |
|
N6- Al38 |
1.864 |
N19- Al47 |
1.812 |
N6- Al51 |
1.816 |
N19- Al43 |
1.815 |
|
N6- Al39 |
1.830 |
N19- Al48 |
1.810 |
N6- Al53 |
1.816 |
N19- Al47 |
1.815 |
|
N6- Si56 |
1.733 |
N19- Al49 |
1.800 |
N6- Al58 |
1.807 |
N19- Al50 |
1.811 |
|
N7- H29 |
1.021 |
N20- Al40 |
1.813 |
N7- Al44 |
1.815 |
N20- Al38 |
1.818 |
|
N7- Al52 |
1.817 |
N20- Al41 |
1.813 |
N7- Al46 |
1.815 |
N20- Al39 |
1.818 |
|
N7- Al54 |
1.806 |
N20- Al47 |
1.803 |
N7- Al53 |
1.811 |
N20- Al43 |
1.814 |
|
N8- Al45 |
1.866 |
N21- Al36 |
1.823 |
N8- Al41 |
1.818 |
N21- H34 |
1.019 |
|
N8- Al54 |
1.850 |
N21- Al37 |
1.813 |
N8- Al42 |
1.818 |
N21- Al55 |
1.815 |
|
N8- Si60 |
1.743 |
N21- Al41 |
1.815 |
N8- Al44 |
1.814 |
N21- Al57 |
1.815 |
|
N9- Al34 |
1.817 |
H22- Al39 |
1.586 |
N9- H32 |
1.019 |
N22- Al52 |
1.816 |
|
N9- Al39 |
1.795 |
H23- Al38 |
1.577 |
N9- Al58 |
1.815 |
N22- Al54 |
1.816 |
|
N9- Al44 |
1.827 |
H24- Al37 |
1.584 |
N9- Al60 |
1.815 |
N22- Al55 |
1.807 |
|
N10- H32 |
1.019 |
H25- Al34 |
1.582 |
N10- Al49 |
1.816 |
N23- Al43 |
1.815 |
|
N10- Al53 |
1.807 |
H26- Al36 |
1.581 |
N10- Al51 |
1.816 |
N23- Al45 |
1.815 |
|
N10- Al54 |
1.815 |
H27- Al35 |
1.582 |
N10- Al60 |
1.807 |
N23- Al52 |
1.811 |
|
N11- Al45 |
1.811 |
Al42- Si57 |
2.414 |
N11- Al44 |
1.815 |
N24- Al39 |
1.818 |
|
N11- Al46 |
1.812 |
Al44- Si55 |
2.403 |
N11- Al48 |
1.815 |
N24- Al40 |
1.818 |
|
N11- Al53 |
1.800 |
Al52- Si59 |
2.386 |
N11- Al51 |
1.811 |
N24- Al45 |
1.814 |
|
N12- Al43 |
1.825 |
Si55- Si56 |
2.326 |
N12- Al37 |
1.818 |
H25- Al42 |
1.582 |
|
N12- Al44 |
1.822 |
Si55- Si60 |
2.311 |
N12- Al42 |
1.818 |
H26- Al41 |
1.582 |
|
N12- Al45 |
1.811 |
Si56- Si57 |
2.334 |
N12- Al48 |
1.814 |
H27- Al40 |
1.582 |
|
N13- Al34 |
1.809 |
Si57- Si58 |
2.303 |
N13- H35 |
1.019 |
H28- Al37 |
1.582 |
|
N13-Al35 |
1.823 |
Si58- Si59 |
2.325 |
N13- Al59 |
1.815 |
H29- Al39 |
1.582 |
|
N13- Al43 |
1.815 |
Si59- Si60 |
2.325 |
N13- Al60 |
1.815 |
H30- Al38 |
1.582 |
Bond angle
Obtained results are shown that by doping of Si-nucleus instead of bond length and bond angle in AlN nanotubes are changed that it`s because of variation of atomic radius. For investigating of bond angles in AlN nanotube, 6 member rings is considered between nanotube in perfect state and different bond angle is considered that is reported in table 2. These results are shown that values of bond angle are similar in same state and is in range of 111.3 to 119.3 degree. But by addition of Silicon nucleus on ring, values of bond angle is varied so much that in ring involving Silicon is varied between 83.9 to 133.6 degree, meanwhile external angles of ring is in range of 88.5 to 124.1 degree. However rings without Silicon nucleus, variation is small in perfect state.
Table 2: Bond angle (Degree) in perfect and Si-dope (6,0) zigzag AlNNTs.
|
Zigzag (6,0) AlNNTs Si-Dope |
Zigzag (6,0) AlNNTs Perfect |
||
|
Bond angle |
Bond angle |
||
|
N6-Si56-Si55 |
114.9 |
N10-Al49-N15 |
119.3 |
|
N6-Si56-Si57 |
111.4 |
N10-Al49-N14 |
118.7 |
|
Al44-Si55-Si60 |
98.0 |
Al48-N15-Al49 |
117.5 |
|
Al44-Si55-Si56 |
88.5 |
Al48-N15-Al47 |
111.3 |
|
N8-Si60-Si55 |
119.3 |
N16-Al47-N15 |
119.3 |
|
N8-Si60-Si59 |
124.0 |
N16-Al47-N19 |
119.3 |
|
Al52-Si59-Si60 |
96.4 |
Al43-N19-Al47 |
111.3 |
|
Al52-Si59-Si58 |
96.3 |
Al43-N19-Al50 |
111.3 |
|
N2-Si58-Si59 |
124.1 |
N18-Al50-N19 |
119.3 |
|
N2-Si58-Si57 |
119.7 |
N18-Al50-N14 |
118.7 |
|
Al42-Si57-Si58 |
97.8 |
Al59-N14-N50 |
117.8 |
|
Al42-Si57-Si56 |
88.9 |
Al59-N14-N49 |
117.8 |
|
Si57-Si58-Si59 |
115.4 |
Al49-N15-Al47 |
117.5 |
|
Si58-Si59-Si60 |
83.9 |
N15-Al47-N19 |
119.2 |
|
Si59-Si60-Si55 |
115.6 |
Al47-N19-Al50 |
117.5 |
|
Si60-Si55-Si56 |
94.6 |
N19-Al50-N14 |
119.3 |
|
Si55-Si56-Si57 |
133.6 |
Al50-N14-Al49 |
112.4 |
|
Si56-Si57-Si58 |
94.9 |
N14-Al49-N15 |
119.3 |
NMR properties
For calculation of NMR parameters, at first all structures are optimized and after that NMR parameters of these structures are considered by GAIO method. For this purpose, chemical shift isotropic and chemical shift anisotropic are considered in different state of structures (Fig 1 and 2).
CSI in zigzag (6,0) AINNTs in perfect and Si-dope states
Obtained results of CSI are shown that electron density values of nucleus aren’t changed and its values are restricted to 221, 183, 173 and 137 ppm. But comparison of this state in Si-dope state is shown that these values are changed by additional of Silicon nucleuses and range of these variations are between 83.13 to 221.36 ppm for Nitrogen nucleuses. However investigation of Aluminum nucleuses are demonstrated these results. CSI value for Aluminum nucleus is in range of 437, 450 and 454 ppm in perfect state and in Si-dope state for Al44 nucleus, CSI value has minimum amount and it is 417.09 ppm. However, in Al48 maximum amount of CSA is 467.73 ppm. For Silicon nucleus, obtained results are proportional to position of Silicon nucleus into nanotube directly (Table 3) . Maybe CSI value is considered as shielding or de-shielding of nucleus. Si58, Si60, Si57 and Si55 are equal in some chemical property and Si59 and Si56 have different chemical property (Fig 3).
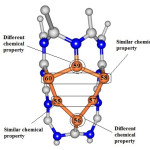 |
Figure 3: Display classify chemical property in 14Si nucleus.
|
Table 3: Isotropic Chemical Shift (ppm) and Anisotropic Chemical Shift (ppm) values for perfect and Si-dope (6,0) zigzag AlNNTs.
|
Zigzag (6,0) AlNNTs |
|||||
|
Perfect |
Si-Dope |
||||
|
Nucleus symbol and number |
CSI |
CSA |
Nucleus symbol and number |
CSI |
CSA |
|
N1 |
221.23 |
30.27 |
N1 |
218.79 |
29.58 |
|
N2 |
183.63 |
28.82 |
N2 |
84.16 |
132.78 |
|
N3 |
173.32 |
27.71 |
N3 |
155.12 |
47.45 |
|
N4 |
137.58 |
67.26 |
N4 |
116.56 |
91.20 |
|
N5 |
221.44 |
28.42 |
N5 |
185.50 |
52.38 |
|
N6 |
183.28 |
30.43 |
N6 |
87.24 |
217.82 |
|
N7 |
173.94 |
26.84 |
N7 |
185.33 |
52.40 |
|
N8 |
137.18 |
67.86 |
N8 |
83.13 |
142.23 |
|
N9 |
221.44 |
28.42 |
N9 |
120.30 |
90.51 |
|
N10 |
183.63 |
28.82 |
N10 |
218.38 |
30.62 |
|
N11 |
173.94 |
26.84 |
N11 |
183.77 |
36.31 |
|
N12 |
137.58 |
67.26 |
N12 |
150.31 |
49.33 |
|
N13 |
221.23 |
30.27 |
N13 |
130.06 |
74.94 |
|
N14 |
183.63 |
28.82 |
N14 |
221.10 |
28.48 |
|
N15 |
173.32 |
27.71 |
N15 |
179.48 |
36.72 |
|
N16 |
137.58 |
67.26 |
N16 |
170.96 |
30.69 |
|
N17 |
221.44 |
28.42 |
N17 |
135.78 |
65.59 |
|
N18 |
183.28 |
30.43 |
N18 |
221.36 |
28.38 |
|
N19 |
173.94 |
26.84 |
N19 |
183.97 |
35.67 |
|
N20 |
137.18 |
67.86 |
N20 |
172.08 |
27.90 |
|
N21 |
221.44 |
28.42 |
N21 |
137.76 |
66.70 |
|
N22 |
183.63 |
28.82 |
Al34 |
436.75 |
22.29 |
|
N23 |
173.94 |
26.84 |
Al35 |
436.88 |
14.29 |
|
N24 |
137.58 |
67.26 |
Al36 |
436.44 |
11.39 |
|
Al37 |
437.37 |
9.08 |
Al37 |
447.87 |
19.67 |
|
Al38 |
437.52 |
9.08 |
Al38 |
443.70 |
80.42 |
|
Al39 |
437.52 |
9.08 |
Al39 |
451.32 |
60.10 |
|
Al40 |
437.37 |
9.08 |
Al40 |
450.64 |
30.03 |
|
Al41 |
437.52 |
9.08 |
Al41 |
458.21 |
29.87 |
|
Al42 |
437.52 |
9.08 |
Al42 |
421.36 |
32.54 |
|
Al43 |
454.53 |
28.30 |
Al43 |
458.46 |
29.52 |
|
Al44 |
454.53 |
28.30 |
Al44 |
417.09 |
50.69 |
|
Al45 |
454.40 |
28.19 |
Al45 |
466.40 |
75.12 |
|
Al46 |
454.40 |
28.19 |
Al46 |
451.11 |
39.55 |
|
Al47 |
454.40 |
28.19 |
Al47 |
451.76 |
39.91 |
|
Al48 |
454.40 |
28.19 |
Al48 |
467.73 |
74.27 |
|
Al49 |
454.54 |
36.33 |
Al49 |
451.26 |
28.45 |
|
Al50 |
454.01 |
36.68 |
Al50 |
453.85 |
49.89 |
|
Al51 |
454.01 |
36.68 |
Al51 |
452.98 |
37.50 |
|
Al52 |
454.01 |
36.68 |
Al52 |
413.05 |
35.99 |
|
Al53 |
454.01 |
36.68 |
Al53 |
451.09 |
27.94 |
|
Al54 |
454.54 |
36.33 |
Al54 |
453.76 |
50.11 |
|
Al55 |
450.48 |
34.89 |
Si55 |
437.28 |
138.16 |
|
Al56 |
450.48 |
34.89 |
Si56 |
102.58 |
453.98 |
|
Al57 |
450.67 |
35.40 |
Si57 |
435.90 |
128.50 |
|
Al58 |
450.67 |
35.40 |
Si58 |
31.22 |
397.57 |
|
Al59 |
450.48 |
34.89 |
Si59 |
405.91 |
187.38 |
|
Al60 |
450.48 |
34.89 |
Si60 |
17.55 |
415.16 |
CSA in zigzag (6, 0) AINNTs in perfect and Si-dope states
Obtained results for CSA values are similar to CSI results and usually CSA values are smaller than CSI. It can be said that by increasing of CSA and CSI values are decreased, on the contrary. Value of this parameter for Nitrogen and Aluminum nucleuses are similar in perfect state, so, for Nitrogen nucleus, CSA values are divided to 27, 28, 30 and 67 ppm. However, for Aluminum nucleuses are 9, 28, 34 and 36 ppm in four different chemical fields. But by doping of Silicon atoms on structure, these variations are increased and based on position of each nucleus to neighbor nucleuses, these variations are complicated. Minimum amount of CSA for nitrogen is 27.90 ppm in N20 and maximum amount of CSA for N6 is 217.82 ppm. However, this series is varied for Aluminum nucleuses and for Al36, minimum amount of CSA is 11.39 ppm and for Al38 nucleuses, maximum amount is 80.42 ppm. For Nitrogen nucleus, obtained results are similar to results of CSI state completely (Table 3).
Conclusion
After theoretical studies in level of theory B3LYP/6-31G(d) on AlN nanotube zigzag (6,0) model in perfect and Si-dope states, obtained results are stated as following:
Values of CSA, CSI, bond lengths and bond angles in perfect state are regular for AlN nanotube structure that is varied by doping of Silicon on nanotube structure and specific value exists for each parameter based on position of Nitrogen, Aluminum and Silicon nucleuses.
CSA and CSI values it`s based on shielded and de-shielded of chemical fields around nucleuses, that in most nucleuses, CSI value is higher than CSA value.
Variation of CSI value for various nucleuses in perfect state, is Aluminum>Nitrogen>Hydrogen and CSA value in 13Al, 1H, 7N nucleuses is close to each other. But by addition of Silicon nucleuses to AlN nanotube, this order is changed, and based on kind of nucleuses. Position of each nucleus in different chemical field, proportional neighbor nucleuses, CSA and CSI have different value.
By addition of Silicon nucleus to annular structure of AlN nanotube, because of variation of atomic, ionic radius, bond length is varied and because regular series of AlN nanotube structure is changed, so angle bond is varied too.
Change of CSA and CSI value is effected on thermal conductivity and electrical conductivity properties that quality of these variations should be considered in future.
Acknowledgements
This work was supported by Islamic Azad University Shahre-rey branch and Islamic Azad University Toyserkan branch.
References
- J. McMurry, Organic Chemistry, 5th Ed., Brooks/Cole, Pacific Grove, CA, 441 (2000).
- A. Seif, A. Boshra, Journal of Molecular Structure: THEOCHEM, 895: 96 (2009).
- M.T. Baei, S.Z. Sayyed-Alangi, A. Soltani, M. Bahari, A. Masoodi, Monatshefte für Chemie / Chemical Monthly, 142: 1 (2011).
- M.T. Baei et al, E-Journal of Chemistry, 8: 609 (2011).
- M. Mirzaei, M. Mirzaei, Journal of Molecular Structure: THEOCHEM, 953: 134 (2010).
- M.T. Baei, A.R. Soltani, P. Torabi, A. Varasteh Moradi, Monatshefte für Chemie / Chemical Monthly, 142: 979 (2011).
- A. Seif, A. Boshra, M. Seif, Journal of Molecular Structure: THEOCHEM, 895: 82 (2009).
- A. Seif, T.S. Ahmadi, A. Bodaghi, J. Hosseini, Journal of Molecular Structure: THEOCHEM, 911: 19 (2009).
- T.S. Ahmadi, A. Seif, G.M. Rozbahani, Arabian Journal of Chemistry, 3: 121 (2010).
- K. Kurotobi, Y. Murata, Science, 333: 613 (2011).
- S. Iijima, Nature, 354: 56 (1991).
- M. Mirzaei, J Mol Model, 17: 89 (2011).
- M. Mirzaei, A. Seif, N.L. Hadipour, Chemical Physics Letters, 461: 246 (2008).
- M. Farahani, T.S. Ahmadi, A. Seif, Journal of Molecular Structure: THEOCHEM, 913: 126 (2009).
- M.J. Frisch et al., GAUSSIAN 09. Gaussian, Inc, Pittsburgh, PA, (2010).

This work is licensed under a Creative Commons Attribution 4.0 International License.









