The Distribution of Selected Metals in the Surface Sediment of Langkawi Coast, Malaysia
M.S. Mohd Zahir¹, B. Akbar John1,2, B.Y. Kamaruzzaman1,2*, K.C.A. Jalal1,2, S. Shahbudin², M. Mohd Fuad1,2, F. Fikriah1,2 and M. Anies Aznida1,2
1Kulliyyah of Science, International Islamic University Malaysia, Jalan Sultan Ahmad Shah, Bandar Indera Mahkota, 25200, Kuantan, Pahang (Malaysia). 2Institute of Oceanography and Maritime Studies, International Islamic University Malaysia, Jalan Sultan Ahmad Shah, Bandar Indera Mahkota, 25200, Kuantan, Pahang (Malaysia).
Surface sediment samples were collected from five stations covering 25 sampling points from the Langkawi coastal waters. Concentration of metals such as Zn, Cu, Pb, Cd, and Cr were determined using an Inductively Coupled Plasma Mass Spectrometry (ICP-MS). The detected concentration range of Zn, Cu, Pb, Cd, and Cr were 41.02-137.1 µgg-1, 14.36-46.32 µg.g-1, 14.4- 38.6 µg.g-1, 0.6-2.4 µg.g-1, and 40.5-84.5 µg.g-1 dry weight respectively. Enrichment factors (EF) analysis showed that the source of Zn, Cu and Cr in the sampling areas were predominantly of terrigenous in origin while the source of Pb and Cd were slightly of anthropogenic in nature.
KEYWORDS:Enrichment Factor; Langkawi Island; Zinc; Copper; Lead; Cadmium; Chromium
Download this article as:| Copy the following to cite this article: Zahir M. S. M, John B. A, Kamaruzzaman B. Y, Jalal K. C. A, Shahbudin S, Fuad M. M, Fikriah F, Aznida M. A. The Distribution of Selected Metals in the Surface Sediment of Langkawi Coast, Malaysia. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Zahir M. S. M, John B. A, Kamaruzzaman B. Y, Jalal K. C. A, Shahbudin S, Fuad M. M, Fikriah F, Aznida M. A. The Distribution of Selected Metals in the Surface Sediment of Langkawi Coast, Malaysia. Available from: http://www.orientjchem.org/?p=11846 |
Introduction
The coastal zone is an important area as it affects the global environments through the interaction between land and ocean (Kremer et al., 2005). Metal concentrations in sediments usually exceed those of the overlying water column (Caroline et al., 2002). As a consequence, metals originating from human activities can often be identified more readily by analysis of sediment than by the quantification of metal concentrations present in water (Haynes et al., 2006). Kevin et al., (2006) has reported the ability of the sediments in integrating the temporal variability of metals that are originating from human sources. He also observed the remobilization of metals during the early stages of post-depositional transformations of the
sediments. Sediments have high physical-chemical stability and their characteristics usually represent the average condition of the system, often being representative of the average water quality (Isaac et al., 2005).
Estuarine and coastal environments are primarily being used as a sinks of river borne metals originating through natural weathering and anthropogenic sources (Hung and Hsu, 2004). Due to the industrialization and urbanization processes, a considerable number of anthropogenic activities such as smelting, mining, electroplating, and other related industrial processes, releases their effluents containing heavy metals into the aquatic water body that ultimately interfere with the natural and ecological equilibrium of the surrounding environment. It should also to be noted that some essential metals (light metals) such as Mn, Fe, Zn, and Cu, are required by aquatic organisms for their proper physiological and biochemical functions. On the other hand, lethal metals such as Hg, As, Cd and Pb, can be toxic to aquatic organisms, even at their trace concentrations. Therefore, types and amounts of metals entering the environment must be considered in assessing possible toxic effects. Hence, the present study was aimed to investigate the metal bioavailability in the surface sediments of Langkawi coastal waters.
Material and Method
Sampling sites
Langkawi is one of the major tourist islands in Malaysia and located in the west coast of Peninsular Malaysia. It is part of Kedah state and governed by the Langkawi Development Authority (LADA). The shores around the main island which consist of soft, silty, and fine sand are protected by several smaller islands surround it. The mean daily temperature in this study area varied from 26.6°C to 29.3°C. Meteorological and bathymetric data showed that the numbers of rain days in a month ranged from none (0) to 27 days and the tides along the Langkawi Island are semi-diurnal type (twice daily) and wind speed was mostly prevailed at 0.3 m/s to 3.3 m/s. Langkawi island is influenced by a wind system (South West Monsoon which blows from May to Sept) that blown from the Indian Ocean which bring monsoon season to Langkawi Island and its adjacent areas.
Samples collection
Five sampling sites were selected along the Langkawi coast covering its entire coast which includes Kuah, Kuala Triang, Pantai Kok, Datai and Tanjung Rhu (Figure 1). The bottom sediment samples were collected using Ekman Grab method (Kamaruzzaman et al., 2011). In order to prevent contamination, the sediments at the top were gently scrapped out and only inner parts were taken. Then, the sediments were transferred into the plastic bottle which has been soak in 5 % nitric acid and were deep freezer prior to analyses. Samples that brought back the laboratory were dried to a constant weigh at 60°C and then sieved under 63µm stainless steel sieves.
 |
Figure 1: Map of study area and sampling point at Langkawi Coastal Waters |
Analytical procedure
The sediment samples were digested and analyzed for the selected metal contents (Zn,Cu, Pb,Cd, and Cr) by followed the published methods (Tsunogai and Yamada, 1979; Noriki et al., 1980; Sen Gupta and Bertnard, 1995; Kamaruzzaman, 1999) with some modifications. Sediment sample (0.05 g) was weighted and heated at 60 ˚C overnight. Then, the sample was kept in a desiccator to cool down. The weight of sample was recorded. These processes were repeated three times to get the constant weight. The sediment sample was transferred into Teflon bomb for closed digestion. 1.5 mL of mixed acid with the ratio of 3.0 (HF): 3.5 (HNO3): 3.5 (HCl) was added and heated at 150 ˚C for 5-7 hours. After heating, the Teflon bomb was cooled down at room temperature. Then, 3.0 mL of boric acid and EDTA were added and heated again at 150 ˚C for 5-7 hours. This mixture was then cooled down at room temperature and a clear solution with no residue was transferred into 10 mL test tube and backed up to 10ml using Milli-Q water. A laboratory standard reference material (Estuarine sediment, SRM 1646a) and a blank reagent were also subjected to the same procedures, in order to determine the precision of the analytical method. The samples then analyzed using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS).
Data analysis
The enrichment factors (EFs) were calculated to evaluate the actual level of elemental contamination by using the earth crust as reference matrix. This analysis would indicate whether the metals are from natural weathering processes of rocks or from anthropogenic sources and reflect status of the environmental contamination. The enrichment of selected heavy metals was calculated using the following formula:
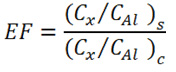
where, Cx and CAl refers to the concentration of elements from the present study (S), and C refers to the crustal value (Taylor, 1964). In this study, the value of earth crust from (Turekian and Wedepohl, 1961; Bowen, 1979; Mason and Moore, 1982) was used as a reference and Al was used as normalizing elements.
Result and Discussion
The concentrations of Zn in surface sediment of Langkawi island ranged from 41.02 to 137.1 µg/g dry weight. The maximum concentration of Zn was found in the Tanjung Rhu samples (132.20 ± 4.62 µg/g dw) while the minimum concentration was recorded at station Kuah (50.12 ± 6.65 µg/g dw). Zn concentration in Pantai Kok, Datai and Kuala Triang was found 102.60 ± 6.82 µg/g dw, 96.30 ± 2.72 µg/g dw and 84.26 ± 3.88 µg/g dw respectively (Figure 3). In general, higher concentrations of Zn in the study areas might be due to its application as an anticorrosive agent in boat paint (Deya et al., 2003), and used as antifouling paint (Konstantinou and Albanis, 2004). Many tourist boat as well as fishing boats in the study areas might also enhanced the Zn level in the environment. Similar observation was noted by (Ismail et al., 1995), who found that heavy metal like Zn in the sediment was higher in the area where boats and shipping activities were dominant. Kamaruzzaman et al., (2006) stated that painting activities of fishing boats and the used of antirust paint in fishing and shipping industries may affect the levels of Zn in the sediments.
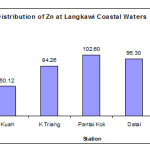 |
Figure 2: Distribution of Zn at Langkawi Coastal Waters. |
Sediment at Kuah station had highest Cu concentration (38.24 μg/g dw). The lowest concentration of Cu was found at Kuala Triang station (18.23 μg/g dw) as shown in Figure 4. The higher concentration of Cu in Kuah was probably due to the following activities being carried out in Kuah station 1. Loading and offloading fishes from fisherman, 2. Cleaning of boats and maintenance, antifouling paint applications, and fuelling activity. WHO, (1998) has reported that these activities would substantially increase the Cu load in an aquatic environment (WHO, 1998). However, in sediment, Cu could be found since it is one of the essential metals for biota, being associated with numerous metalloenzymes and metalloproteins (Kamaruzzaman et al., 2011). However, high level of Cu in the sediment could be potentially toxic and this may pose concern because of bioaccumulation once in the food chain. The high level of Cu found in the sediment could be due to both natural origins and man-induced activities which are widely reported in the literature (Christensen and Juracek, 2001).
Lead (Pb) concentration was highest in Kuala Triang (33.19 μg/g dw) and lowest at Tanjung Rhu (15.54 μg/g dw). Concentration of Pb in Kuah and Kuala Triang stations were comparable with the previous study carried out by Wan Mohd Razi et al., (2009), who recorded the Pb concentration of 35 ± 16.17 μg/g dry weights. Compared to our study, there is a slight reduction in the Pb concentration in Kuah station was apparent during 2009 though it is not statistically significant (p > 0.05). Higher concentrations of Pb as the consequence of the usage of leaded gasoline in ships and boats as well as spillage during shipment and other operations that had took place along the area (Kamaruzzaman et al., 2008). Activities such as cleaning and painting of boats would also contribute higher concentrations of Pb into the environment. Beside that, emissions from automobiles that consume leaded petrol are the major source of the atmospheric Pb, as the atmospheric route may represent a major source of Pb into the marine environment (Jalal et al., 2009).
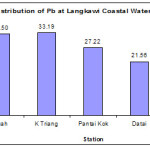 |
Figure 4: Distribution of Pb at Langkawi Coastal Waters. |
Station Kuah had the highest concentration of Cd (1.10 ± 0.47 µg/g dw), followed by Kuala Triang (0.84 ± 0.30 µg/g dw), Datai (0.58 ± 0.16 µg/g dw), Pantai Kok (0.43 ± 0.12 µg/g dw) and Tanjung Rhu (0.34 ± 0.12 µg/g dw) (Figure 6). Cadmium concentration in Langkawi coastal sediments ranged between 0.6 and 2.4 µg/g dry weights. According to Lindsey et al., (2004), levels of Cd depend on industrial activities, fishing landing ports, domestic sewage and Cd concentrations are usually high in the estuary region. Field observation showed that a lot of boats that bring passengers and tourist pass through the area everyday which might influence the Cd load in the environment. Besides this fact, Cd is a component of petrol’s, diesel fuels and lubricating oil. Seepage as well as spillage of fuel during shipment process would also results in increasing of Cd levels into the adjacent area such as Kuah station.
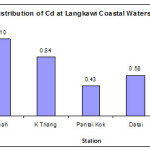 |
Figure 5: Distribution of Cd at Langkawi Coastal Waters |
The concentrations of Cr in Langkawi coastal sediment ranged from 40.5 – 84.5 µg/g dry weights. The highest and lowest concentration of Cr was found at Kuala Triang station (76.15 ± 6.48 µg/g dw) and Datai (45.65 ± 3.41 µg/g dw) respectively (Figure 7). Higher concentrations of Cr are most probably caused by anthropogenic activities. According to Ahmed et al., (2007) and Lindsey et al., (2004), a variety of anthropogenic activities could contribute to the release of Cr to the environment. Cr released by the electroplating activities, steel manufacturer, leather tanning, and textile industries is also a large responsible of contamination in this study area. However, the absence of such industries along the Langkawi Island proved that the source of Cr is from various inshore fishery activities. This observation was well corresponded with the recent study conducted by Cuong et al., (2008), who noted that the inshore marine activities such as shipment, discharge of oil at the sea, application of antifouling paint and anti corrosive paint, oil spills during shipment to the residence also will results in higher concentrations of Cr. High concentration of Cr in the study area might be due an intensive usage of an antirust paint and anticorrosive paint that contains Cr element
(Kamaruzzaman et al., 2008). In addition, higher concentrations of Cr obtained from this study might also caused by the applications of fertilizers that were used in agriculture activities (Teck et al., 2010).
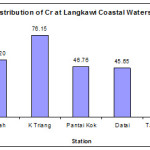 |
Figure 6: Distribution of Cr at Langkawi Coastal Waters.
|
Table 1: Mean EF value determined in sediment of Langkawi coastal water
|
Element |
EF value |
Contamination category |
|
Cr |
1.83 ± 0.23 |
Deficiency to minimal enrichment |
|
Cu |
1.34 ± 0.41 |
Deficiency to minimal enrichment |
|
Zn |
1.89 ± 0.08 |
Deficiency to minimal enrichment |
|
Cd |
2.77 ± 0.41 |
Moderate enrichment |
|
Pb |
4.95 ± 0.47 |
Moderate enrichment |
Table 1 shows the enrichment factor (EF) values of selected heavy metals in Langkawi coastal water. In this study, the enrichment of the metals over the crustal values was in the order of Pb > Cd > Zn > Cr > Cu. The slightly higher proportion of Pb and Cd implies that the sediments are contaminated by the atmospheric deposition of finer particles, domestic effluent discharges and the extensive use of paints along the study area (Selvaraj et al., 2004). Though the sources of other metals such as Zn, Cr and Cu are high in the study area, their minimal enrichment value showed that these metals concentration in Langkawi coastal sediment probably do not cause much effect on the organisms living in the coastal sediment bed. Nevertheless, long term environmental monitoring program would give crucial details on the quality of the Langkawi coastal areas as these metals affects the distribution of sensitive fauna along the coastal region.
Acknowledgements
This research was conducted with joint funding from the International Islamic University Malaysia under the Research Matching Grant Scheme (RMGS) and Grant from the Sultan Qaboos University, Oman. The authors wish to express their gratitude to the Lab Assistant laboratory teams of both the universities for their invaluable assistance throughout the sampling period.
References
- Kremer, H.H., Le Tissier, M.D.A., Burbridge, P.R., Talaue-McManus, L., Rabalais, N.N., Parslow, J., Crossland, C.J., & Young, B. Editors, Land–Ocean Interactions in the Coastal Zone: Science Plan and Implementation Strategy. IGBP Report 51/IHDP Report 18, IGBP Secretariat, Stockholm. (2005)
- Caroline, C., Lyn, C. N., Iain, W., & David, J.. Concentrations of Cd, Zn and Cu in sediments and brown shrimp (Crangon crangon L.) from the Severn Estuary and Bristol Channel, UK. Marine Environmental Research 54, 331-334 (2002)
- Haynes, D., Toohey, D., Clarke, D., & Marney, D.. Temporal and spatial variation in concentrations of tracemetals in coastal sediments from the Ninety Mile Beach, Victoria, Australia, Marine Pollution Bulletin. 30: 414–418. (1995)
- Kevin H. T.,Bahram D.,Michael S.,Deborah, K.P., & Denis G. R.. The role of smelter emissions and element remobilization in the sediment chemistry of 99 lakes around the Horne smelter, Québec .The Geological Society 6:187-202. (2006)
- Isaac R. S., Emmanoel V. S., Carlos E.G.R., Schaefer, Manoel R., Albuquerque, F., & Lúcia S. C.. Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Marine Pollution Bulletin 50(2):185-194. (2005)
- Hung, J.J & Hsu, C.L. Present state and historical changes of trace metal pollution in Kaoping coastal sediments, southwestern Taiwan. Marine Pollution Bulletin 49; 986-998. (2004)
- Kamaruzzaman, B.Y., N.T. Shuhada, B. Akbar, S. Shahbudin, K.C.A. Jalal, M.C. Ong, S.M. Al-Barwani and & J.S. Goddard. Spatial Concentrations of Lead and Copper in Bottom Sediments of Langkawi Coastal Area, Malaysia. Research Journal of Environmental Sciences, 5: 179-186. (2011).
- Tsunogai, S. & Yamada, M.. 226Ra in Bering Sea sediment and its application as a geochronometer. Geochemical Journal. 13: 231-238. (1979).
- Noriki, S., K. Nakanishi, T. Fukuwa, M. Uematsu, T. Uchida & S. Tsunogai. Use of a teflon vessel for the decomposition followed by the determination of chemical constituents of various marine samples. Bull. Fac. Fish, Hokkaido Univ., 31, 354-465. (1980)
- Sen Gupta, J.G., Bertnard, N.B.. Direct ICP-MS Determination of trace and Ultratrace Elements in Geological Materials after Decomposition in a Microwave Oven, Quantitation of Y, Th, U and Lanthanides. Talanta. 42,1595-1607. (1995)
- Kamaruzzaman, B.Y. Geochemistry of the Marine Sediments: Its Paleoceanographic Significance. D.Sc Thesis. Hokkaido University, Japan. (1999)
- Taylor, S. R. Abundance of chemical elements in the continental crust: A new table. Geochimica Cosmochimica Acta. 28: 1273 – 1285. (1964)
- Turekian, K. K. & Wedepohl, K.H. Distribution of the elements in some major units of the earth’s crust. Bull. Geol. Soc. Am. 72: 175–192. (1961)
- Bowen, H. J. M. Environmental Chemistry of the Elements. London: Academic Press. (1979).
- Mason, B. & Moore, C. B. Principles of Chemistry, England (4th Edition): J. Wiley and Sons. (1982).
- Deya, M., Vetere, VF., Romagnoli, R., Del Amo, B. Zinc Tripolvphosphate: An Anticorrosive Pigment for Paints.” Surf. Coat. Int., 86 (B1) 79-85. (2003)
- Konstantinou, I.K. & Albanis, T.A. World-wide occurrence and effects of antifouling paint booster biocides in the aquatic environment: A review Environment International. 30 (2), 235- 248. (2004)
- Ismail, A., Jusoh, N.R., & Ghani, I.A.. Trace metal concentrations in marine prawns off the Malaysian coast. Marine Pollution Bulletin, 31(1-3):108-110. (1995)
- Kamaruzzaman, B. Y., Shazili, N. A. M., Willison, K. Y. S., Ong, M. C. & Norhizam, H. A. G. The role of northeast monsoon seasons in the dilution of heavy metal concentrations in sediments off Pahang, South China Sea. Human Health Risks and Marine Environment Quality ICES CM G: 01. (2006)
- WHO Copper: Environmental Health criteria Monograph 200. International Program on Chemical Safety (IPCS), (1998).
- Christensen, V. G., & Juracek, K. E. Variability of metals in reservoir sediment from two adjacent basins in the central Great Plains. Environmental Geology. 40: 470-481. (2001).
- Wan Mohd Razi, I., Sahibin, A.R., Zulfahmi, A.R., Tukimat, L., Azman, H., & Shahril Nizam, M. Y. Geochemical Composition of Beach Sediment in Langkawi Island, Kedah, Malaysia. Sains Malaysiana. 38(3): 313–320. (2009).
- Kamaruzzaman, B.Y., Ong, M.C., Noor Azhar, M.S., Shahbudin, S., & Jalal, K.C.A. Geochemistry of sediment in the major estuarine mangrove forest of Terengganu region, Malaysia. Am. J. Appl. Sci. 5:1707-1712. (2008).
- Jalal, K. C. A., Noor Faizul, H. N.,Kamaruzzaman, B. Y., Shahbudin, S., Alam, M. Z. & Jaswir Irwandi. Studies on physico-chemical characteristics and sediment environment along the coastal waters in Pulau Tuba, Langkawi, Malaysia. Aquatic Ecosystem Health and Management. 12(4): 350-357. (2009).
- Lindsey, H. D., James, M. M., & Hector, M. G. An assessment of metal contamination in mangrove sediments and leaves from Punta Mala Bay, Pacific Panama. Marine Pollution Bulletin 50 (2005) 547–552. (2004).
- Ahmed El Nemr, Tarek, O., Said, Azza Khaled, Amany El-Sikaily & Aly, M.A. Abd-Allah. The distribution and sources of polycyclic aromatic hydrocarbons in surface sediments along the Egyptian Mediterranean coast. Environ. Mon. Assess. 124 (1-3) 343-359. (2007).
- Cuong, D.T., Karuppiah, S., & Obbard, J.P. Distribution of Heavy Metals in the Dissolved and Suspended Phase of the Sea Surface Microlayer, Seawater Column and in the Sediments of Singapore’s Coastal Enviroment. Environmetal Monitoring Assessment. 138: 255-272. (2008).
- Teck,Y. L., Rohene, S., Chui, P.K., & Nyanti, L. Organic Matter, Nutrients and Trace Metals of Serin River. World Applied Sciences Journal 8 (4): 496-502, 2010. (2010).
- Selvaraj, K.,Ram Mohan, V.,& Piotr S, Evaluation of metal contamination in coastal sediments of the Bay of Bengal, India: geochemical and statistical approaches. Marine Pollution Bulletin 49 (3): 174-185. (2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.









