Synthesis of N-Arylisatins Using Nay Heterogeneous Catalyst Under Microwave Irradiations
Ravinder Singh* and Ramesh Kumar
Department of Chemistry, Pt.N.R.S.Govt. College, Rohtak-124 001,Haryana, (India).
N-Arylisatins are synthesized in high yield in shorter reaction time by the reaction of 2-oxo-2-(Arylamino)acetates and arynes using NaY heterogeneous catalyst under microwave irradiations.
KEYWORDS:Catalyst; Microwave; Heterogeneous catalyst; N-Arylisatins
Download this article as:| Copy the following to cite this article: Singh R, Kumar R. Synthesis of N-Arylisatins Using Nay Heterogeneous Catalyst Under Microwave Irradiations. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Singh R, Kumar R. Synthesis of N-Arylisatins Using Nay Heterogeneous Catalyst Under Microwave Irradiations. Available from: http://www.orientjchem.org/?p=23541 |
Introduction
Isatin have both biological and medical properties including antifungal, antiviral, anti-HIV, anti-, AIDS, antiprotozoal, anticancer and antileukema.1-7
In recent years, Isatin have been synthesized by different methods but they require tedious work up and long reaction time.8-17 Microwave assisted organic synthesis is currently gaining ground in synthetic organic chemistry largely due to the dramatic reduction in time (from days or hours to minutes or seconds).18-19
As a part of our ongoing research “roll of microwave technique in synthesis of organic compounds in presence of zeolites”, we report a microwave assisted synthesis of N-Arylisatin by reaction of Arynes with Methyl-2-oxo-2-(arylamino)acetate. Excellent yield of N-Arylisatins were achieved in shorter time.
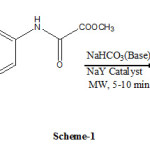 |
Scheme 1 Click here to View scheme |
Table 1: Reaction of various Methyl 2-oxo-2(aryl amino)acetates with 2-(Trimethylallyl)phenyl Trifluoromethanesulfonate
| S.No. | Substrate(R2) |
Time (Minutes) |
Yield (%) |
M.pt. (OC) |
|
|
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 |
H 4-Me 4-Oph 4-F 4-Cl 4-Br 4-I 2-1 4-CN 4-CO2Et 4-CF3 3-CF3 2-t-Bu 2-Ph 2,5-(Ome)2 2,4,6-Me3 2,3-(CH)4 |
7 6 5 6 7 7 7 7 4 10 8 9 8 8 5 5 5 |
91 95 95 97 81 83 87 94 86 88 71 75 94 89 91 82 98 |
136-139 137-139 145-148 235-237 194-197 178-180 198-201 173-176 282-284 127-129 177-181 124-127 109-113 157-162 114-117 164-168 130-133 |
|
Result and discussion
we started our work by reacting methyl-2-oxo(phenyl amino)acetate with 2-(trimethylallyl)phenyltrifluoromethanesulphonat in presence of NaY zeolite under microwave irradiation selected power(400W) for 7 minutes. We obtained N-phenylisatin in high yield(91) in lower reaction time (7min). The probable reaction mechanism is:
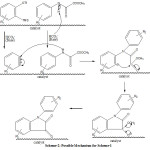 |
Scheme 2 |
Take another compound and observed percentage yield and other properties. No side product is obtained by controlling temperature in the temperature monitoring microwave oven. Acetonitrile was the best solvent used for this reaction. Here electron-rich substituent seems to promote this reaction to a small extent, whereas electron deficient group lower the yield of the reaction in same way as in ordinary conditions.
It is believed that two mechanisms are possible for this process, routes A and B in Scheme 3. Both suggested rouetes share a common first step, nucleophilic attack by nitrogen on the benzyne, resulting in an Aryl carbanion. From there, at least two posibilities exist with regard to how the desired isatin is formed. Route Asuggests that the Aryl carbanion attacks the distant ester carbony and displaces a methoxy group. This type of mechanism has also been suggested in our earlier work. On the other hand, Greany’s work(eq. 1) suggests another possibility, route B. This route involves attack of the aryl carbanion onto thenearest caronyl group, the amide carbonyl, forming a strained four-membered ring intermediate, which then fragments into a structure where the nitrogen can now attack the ester carbonyl. Both routes eventually lead to the desired isatin. However, the carbonyl groups end up in different places depending on the route. Further efforts are underway in order to shed light on this process.
Experimental section:
General. The 1H and 13C NMR spectra were recorded at 300 and 75.5MHz or 400 and 100MHz, respectively. Thin layer chromatography was performed using commercially prepared 60-mesh silica gel plates, and visualization was effected with short wavelength UV light(254nm). All melting points uncorrected. All reagents are used directly as obtained commercially.
Typical procedure for synthesis of the Isatin derivatives 1-17. To a dry s dram vial containing a solution of the amide (0.5mmol) and the aryn precursor (1.0mmol) in MeCN (5.0ml, anhydrous) was added NaHCO3 (1.0mmol) and CsF (3.0mmol). The vial was sealed and allowed to stir for 24H at rt. The reaction mixture was then filtered through a lug of silica gel using ethyl acetate, concentrated in vacuo, and purified by flash chromatography using gradient solvent combinations of ethyl acetate/Hexanes or dichloromethane/Hexanes.
1-Phenylindoline-2,3-dione(1): The product was isolated as an orange solid.1H NMR (300MHz,CDCl3)δ 7.70 (ddd,j=0.6, 1.3, 7.5Hz,1H), 7.60-7.41(m, 6H), 7.18(td,j= 0.8,7.6Hz, 1H), 6.91(d,j= 8.1Hz, 1H), 13C NMR (75MHz,CDCl3) δ 183.1, 157.5, 151.9, 138.5, 133.4, 130.2, 129.0, 126.2, 125.8, 124.5, 117.5; HRMS(EI) calcd for [M+H]+ (M=C14H9NO2) 224.0706, found 224.0707
1-p-Tolylindoline-2,3-dione(2): The product was isolated as a red-orange solid.1H NMR (400MHz,CDCl3)δ 7.80 (d,j=7.5Hz,1H), 7.66(t,j=7.3Hz,1H), 7.49(d,j= 8.2Hz,2H), 7.42(d,j= 8.3Hz, 2H), 7.30(d,j=7.5Hz,1H) 7.00(d,j=8.1Hz,1H) 2.56(s,3H), 13C NMR (100MHz,CDCl3) δ 183.2, 157.6, 152.0, 139.1, 138.5, 130.7, 130.3, 126.0, 125.6, 124.3, 117.6, 111.4, 21.4; HRMS(EI) calcd for C18H11NO2 237.0790, found 237.0791
1-(4-phenoxyphenyl)indoline-2,3-dione(3): The product was isolated as a yellow-orange solid.1H NMR (400MHz,CDCl3)δ 7.66 (d,j=7.4Hz,1H), 7.54(t,j=7.8Hz,1H), 7.40-7.34(m,4H), 7.18-7.11(m,4H), 7.07(d,j= 8.2Hz,2H), 6.88(d,j= 7.97Hz, 1H), 13C NMR (100MHz,CDCl3) δ 183.0, 157.9, 157.6, 156.2, 151.8, 138.5, 130.1, 127.7, 127.4, 125.6, 124.4, 124.3, 119.8, 119.5, 117.5, 111.3, HRMS(EI) calcd for C20H13NO3 315.0895, found 315.0899
1-(4-Fluorophenyl)indoline-2,3-dione(4): The product was isolated as a bright orange solid.1H NMR (400MHz,CDCl3)δ 7.72(d,j=7.5Hz,1H), 7.57(t,j=7.8Hz,1H), 7.44-7.40(m,2H), 7.29-7.24(m,2H), 7.20(t,j= 7.6Hz,1H), 6.86(d,j= 8.0Hz, 1H), 13C NMR (100MHz,CDCl3 extra peaks due to C-F coupling) δ 182.8, 163.7, 161.2, 157.6, 151.7, 138.6, 129.0, 128.9, 128.3, 128.2, 125.9, 124.7, 117.7, 117.4, 117.2, 111.3, HRMS(EI) calcd for C14H8FNO2 241.0539, found 241.0539
1-(4-Chlorophenyl)indoline-2,3-dione(5): The product was isolated as an orange solid.1H NMR (400MHz,CDCl3)δ 7.71(d,j=7.5Hz,1H), 7.59-7.53(m, 3H), 7.39(d,j=8.6Hz, 2H), 7.20(t,j=7.6Hz,1H), 6.90(d,j=6.9Hz, 1H); 13C NMR (100MHz,CDCl3) δ 128.6, 157.4, 151.3, 138.6, 134.7, 131.5, 134.4, 137.5, 126.0, 124.7, 117.7, 111.3 HRMS(EI) calcd for C14H8ClNO2 257.0244, found 257.0248
1-(4-Bromophenyl)indoline-2,3-dione(6): The product was isolated as a light orange solid.1H NMR (400MHz,CDCl3)δ 7.69-7.66(m,3H) 7.56(t,j=7.8Hz, 1H), 7.32(d,j=8.5Hz,2H), 7.20(t,j=7.5Hz, 1H), 6.90(d,j=8.0Hz, 1H); 13C NMR (100MHz,CDCl3) δ 182.5, 157.2,. 151.1, 138.6, 133.3, 132.0, 127.7, 125.9, 124.7, 122.6, 117.6, 111.3, HRMS(EI) calcd for C14H8BrNO2 300.9738, found 300.9742
1-(4-Iodophenyl)indoline-2,3-dione(7): The product was isolated as an orange solid.1H NMR (400MHz,CDCl3)δ 7.88(d,j=8.5Hz, 2H), 7.69(d,j=7.51Hz, 1H), 7.56(t,j=7.8Hz,1H), 7.21-7.17(m, 3H), 6.90(d,j=8.0Hz, 1H); 13C NMR (100MHz,CDCl3) δ 182.5, 157.2, 151.1, 139.3, 138.6, 132.7, 127.8, 126.0, 124.8, 117.6, 11.3, 94.1; HRMS(EI) calcd for C14H8INO2 348.9600, found 348.9601
1-(2-Iodophenyl)indoline-2,3-dione(8). The product was isolated as a ruby red solid.1H NMR(400MHz,CDCl3) δ 8.01(d,j=7.9Hz,1H), 7.70(d,j=7.4Hz, 1H), 7.53-7.51(m, 2H), 7.36(d,j=7.6Hz, iH), 7.26-7.15(m, 2H), 6.49(d,j=8.0Hz, 1H); 13C NMR(100MHz,CDCl3)δ 182.6, 157.0, 151.3, 140.8, 138.7, 136.2, 131.5, 130.2, 129.7, 125.8, 124.5, 117.2, 111.8, 98.1; HRMS(EI) calcd for [M+Na]+ (M=C14H8INO2) 371.9492,found 371.9497
4-(2,3-dioxoindolin-1-yl)benzonitrile(9): The product was isolated as a light orange solid.1H NMR (400MHz,CDCl3)δ 8.08(d,j=8.3Hz, 2H), 7.714-7.705(m, 3H), 7.64(t,j=7.8Hz, 1H), 7.23(t,j=7.51Hz,1H), 6.99(d,j=7.9Hz, 1H); 13C NMR (100MHz,CDCl3) δ 181.9, 157.2, 150.0, 137.9, 137.6, 133.8, 127.0, 124.9, 124.1, 118.4, 117.9, 110.9, 110.5,; HRMS(EI) calcd for [M+H]+(M=C15H8N2O2) 249.0659, found 249.0656
Ethyl-4-(2,3-dioxoindolin-1-yl)benzoate(10): The product was isolated as a light orange solid.1H NMR (400MHz,CDCl3)δ 8.21(d,j=8.6Hz, 2H), 7.70(d,j=7.5Hz, 1H), 7.59-7.52(m, 3H), 7.20(t,j=7.51Hz,1H), 6.97(d,j=8.1Hz, 1H), 4.40(d,j=7.1Hz, 2H), 1.41(t,j=7.1Hz, 3H) 13C NMR (100MHz,CDCl3) δ 182.3, 165.6, 157.1, 150.9, 138.6, 136.9, 131.3, 130.6, 126.0, 125.5, 124.8, 117.7, 111.4, 61.5, 14.5; HRMS(EI) calcd for C17H13NO4 295.0839, found 295.0845
1-[4-(Trifluoromethyl)phenyl]indolin-2,3-dione(11): The product was isolated as a bright orange solid.1H NMR (400MHz,CDCl3)δ 7.84(d,j=8.4Hz, 2H), 7.73(d,j=6.9Hz, 1H), 7.62-7.59(m, 3H), 7.23(t,j=7.5Hz,1H), 6.98(d,j=8.1Hz, 1H); 13C NMR (100MHz,CDCl3, extra peaks due to C-F coupling) δ 182.2, 157.2, 150.8, 138.7, 136.2, 131.0, 130.6, 128.9, 127.33, 127.29, 127.26, 127.22, 126.3, 126.1, 125.1, 125.0, 122.4, 117.7, 111.3, HRMS(EI) calcd for C15H8F3NO2 291.0507, found 291.0509
1-[3-(Trifluoromethyl)phenyl]indolin-2,3-dione(12): The product was isolated as a yellow orange solid.1H NMR (400MHz,CDCl3)δ 7.74-7.65(m, 5H), 7.60(td,j=1.2,8.0Hz, 1H), 7.23(t,j=7.6Hz, 1H), 6.92(d,j=8.1Hz, 1H); 13C NMR (100MHz,CDCl3, extra peaks due to C-F coupling) δ 182.3, 157.3, 150.9, 138.7, 133.7, 130.9, 129.6, 126.1, 125.74, 125.70, 125.0, 123.04, 123.00, 117.7, 111.2; HRMS(EI) calcd for C15H8F3NO2 291.0507, found 291.0507
1-(2-tert-Butylphenyl)indolin-2,3-dione(13): The product was isolated as a light orange solid.1H NMR (400MHz,CDCl3)δ 7.66(t,j=7.9Hz, 2H), 7.51(td,j=1.1, 7.8Hz, 1H), 7.44(t,j=7.7Hz, 1H), 7.34(td,j=1.3,7.5Hz, 1H), 7.14(t,j=7.5Hz, 1H), 7.05(dd,j=1.4,7.8Hz, 1H), 6.42(d,j=8.0Hz, 1H), 1.32(s, 9H); 13C NMR (100MHz,CDCl3, δ 183.4, 159.2, 154.1, 149.6, 138.7, 131.7, 130.5, 130.2, 129.4, 128.4, 125.4, 124.2, 11.8, 112.7, 35.8, 31.9; HRMS(EI) calcd for C18H17NO2 279.1259, found 279.1259
1-((1,1/-Biphenyl)-2-Yl]indoline-2,3-dione(14). The product was isolated as a red solid.1H NMR(400MHz,CDCl3) δ 7.56-7.53(m,4H), 7.40-7.36(m,2H), 7.26-7.23(m,5H), 7.03(t,j=7.3Hz,1H), 6.48(d,j=7.5Hz,1H), 13C NMR(100MHz,CDCl3)δ 182.8, 158.0, 152.2, 141.1, 138.3, 138.2, 131.7, 130.6, 130.1, 129.3, 128.74, 128.69, 128.2, 128.1, 125.4, 124.1, 117.2, 111.6, HRMS(EI) calcd for C30H11NO2 299.0946, found 299.0946
1-(2,4-Dimethoxyphenyl)indoline-2,3-dione(15). The product was isolated as a orange solid.1H NMR(400MHz,CDCl3) δ 7.64(dd,j=7,7.5Hz,1H), 7.49(t,j=1.4,7.9Hz,1H), 7.11(td,j=0.73,7.5Hz, iH), 7.02-6.96(m,2H), 6.86(d,j=2.6Hz,1H), 6.59(d,j=8.0Hz,1H), 3.77(s, 3H), 3.73(s, 3H); 13C NMR(100MHz,CDCl3)δ 183.1, 157.7, 154.0, 152.2, 149.3, 138.4, 125.3, 124.0, 121.6, 117.6, 116.0, 114.7, 113.7, 111.8, 56.4, 56.0; HRMS(EI) calcd for C16H13NO4 283.0845 found 283.0839
1-Mesitylindoline-2,3-dione(16). The product was isolated as a yellow orange solid.1H NMR(400MHz,CDCl3) δ 7.70(d,j=7.2Hz,1H), 7.51(t,j=7.7Hz, 1H), 7.16(t,j=7.5Hz, iH), 7.03(s,2H), 6.4(d,j=7.8Hz,1H), 2.35(s, 3H), 2.14(s, 6H); 13C NMR(100MHz,CDCl3)δ 13.2, 157.5, 151.7, 139.8, 138.8, 136.3, 129.9, 128.0, 125.8, 124.3, 117.7, 111.2, 21.3, 18.1; HRMS(EI) calcd for C17H15NO2 265.1100 found 265.1103
1-(Naphthalen-1-yl)indoline-2,3-dione(17). The product was isolated as an orange solid.1H NMR(400MHz,CDCl3) δ 8.02(d,j=8.2Hz,1H), 7.98(d,j=8.2Hz, 1H), 7.75(d,j=7.3Hz, iH), 7.70(t,j=8.4Hz, 1H), 7.64-7.49(3,4H), 7.45(td,j=1.1,7.9Hz,1H), 7.17(t,j=7.5Hz, 1H), 6.44(d,j=8.0Hz, 1H), 6H); 13C NMR(100MHz,CDCl3)δ 183.1, 158.2, 152.8, 138.8, 135.0, 130.4, 129.5, 129.0, 127.6, 127.1, 126.0,. 125.7, 124.4, 122.6, 117.6, 112.0; HRMS(EI) calcd for C18H11NO2 273.0790 found 273.0786
Refrences
- (a) For a review, seey Pandeya, S, N Samitha, S Jyoti , M Sridhar, S.K Acta Pharm, 2005, 55, 27. (b) Vine, K.L; Matesh, L Locke, J.M Ranson, M kropeta, D. Anti cancer agents, Med Chem. 2009,9,397. (c) Shultleworth, S.J, Nasturica, D Gervais, C Siddiqui, M.A Rando, R.F Lee, N. Bioorg. Med. Chem. Lett. 2000, 10, 250.
- Islam, M.R Abedin, M.JHossain, M.M Doddeck, H.J. Bangladesh Chem. Soc. 1998, 11, 71.
- Kilpin, K.J Henderson, WNicholson, B.K. Polyhedron 2007, 26, 2004.
- Islam, M.RAbedin, J Khayer, K. Indian J. Chem. 2001, 40, 240.
- Varma, R.SKhan, I.A. Polish J. Pharmacol. Phann 1977, 29, 549.
- Li, CGo, Y Mao, Z Koyano, K Kai, Y Kanesha, N Zhu, QZho, Z Wu, S.Bull Chem. Soc. Jpn. 1996, 69, 1621.
- Mcnell, T.A. Antimicrob. Agents Chemother, 1973, 4, 105.
- (a) Romagnoli, R; Baraldi, P.G; Cruz-Lopez, C Preti, D Bermejo, J Estavez, F. Chem. Med. Chem. 2009, 4, 1668. (b) Bursavich, M. G Gilbert, A.M Lombardi, S Georgiadis, K. E; Reifenberg, E4 Flannery, C. R; Morris, E.A. bioorg. Med. Chem. Lett. 2007,17, 5630. (c) Konkel, M.J Packiarajan, M Chem. H Topiwala, U.P4 Jimenez, H Talisman, I.J Coate, H Walker, M.W. Bioorg. Med. Chem. Lett, 2006, 16, 3950. (d) Lam, P.Y.S Vinoent, G Clark, C.G Dcudon, S Jadhav, P.K. Tetrahedron Lett, 2001, 42, 3415.
- (a) Shindikar, A.V Khan, F Viswanathan, C.L. Eur.J.Med. Chem. 2006,41, 786. (b) Moser, P4 Sallmann, A Wieserberg, I.J. Med. Chem. 1990, 33, 2358. (c) Sarges, R Howard, H. R Koe. H.K Weissman, A.J.Med. Chem. 1989, 32, 437.
- Peet, N.J. Heterocycl, Chem. 1990, 17, 1514.
- For reviews, see: (a) Chem, Y Larock, R.C. In Modern Arylation Methods, Ackerman, J. ED Wiley/ VCH New York, 2009, PP401. (b) Sanz, R.Org. Prep. Proced. Int. 2008, 40, 215.
- (a) Lin, Z; Larock, R. C. J. Org. Chem. 2006, 71, 3198. (b) Lin, Z; Larock, R. C. Org. Lett. 2003, 5, 4673. (c) Lin, Z; Larock, R. C. Org. Lett. 2004, 6, 99.
- Lin, Z; Larock, R. C. J. AR.Chem. Sec. 2005, 127, 13112.
- Pintori, D. G Greaney, M.F.Org. Lett. 2010, 12, 168.
- Yoshida, H Shirakawa, E4 Honda, Y Hiyama, T. Angew. Chem, Inted. 2002, 41, 3246.
- Zhao, J Larock, R.C.J.Org. Chem. 2007, 72, 583.
- Rogness D. C Larock, R.C. Tetrahedron Lett 2009, 50, 4003.
- Lin, Z; Larock, R.C.J. Org. Chem. 2006, 71, 3198.
- Peddibhotla, S. Curr. Bioact. Compd. 2009, 5, 20.

This work is licensed under a Creative Commons Attribution 4.0 International License.









