Self-Assembling Characteristics of Cefuroxime Axetile - An Antibiotic Agent
Pinki Sharma and L. K. Tiwary*
Regional Institute of Education, NCERT, Bhopal - 462 001, India.
Cefuroxime Axetile (CA) is a semi-synthetic broad-spectrum cephalosporin antibiotic for oral administration. The critical micelle concentration (CMC) of this drug determined by spectrophotometric method is found to be 0.2 mM and this value decreases in presence of sodium dodecyl sulphate (SDS) and cetyltrimethylammonium bromide (CTAB). The CMC of CA decreases from 0.2 to 0.12 mM by increasing the concentration of SDS from 0.05 to 5.0 mM. This trend is not regular with CTAB. There is remarkable decrease in CMC of CA from 0.2 to 0.15 mM by increasing concentration of CTAB up to 0.1 mM beyond which it acquires a constant value of 0.14 mM. The effect of CA on micellization of SDS and CTAB has also been investigated in detail in the premicellar region of drug. The CMC of SDS decreases from 7.8 mM to 2.5 mM by increasing the concentration of CA from 0.02 to 0.1 mM. Similar trend has also been observed in case of CTAB where its CMC decreases from 0.86 mM to 0.27 mM within the same concentration range of CA. Effect of temperature on micellization of SDS - CA and CTAB – CA mixed micellar systems has been studied at 303, 313 and 323 K. Physicochemical parameters have been calculated using biphasic model.
KEYWORDS:Cefuroxime Axetile; Antibiotic agent; Spectrophotometric method
Download this article as:| Copy the following to cite this article: Sharma P, Tiwary L. K. Self-Assembling Characteristics of Cefuroxime Axetile - An Antibiotic Agent. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Sharma P, Tiwary L. K. Self-Assembling Characteristics of Cefuroxime Axetile - An Antibiotic Agent. Available from: http://www.orientjchem.org/?p=23538 |
Introduction
Amphiphilic compounds (amphiphiles) having polar and nonpolar characteristics are surface active. They are preferentially adsorbed at the air / liquid, liquid / liquid, liquid / solid and solid / solid interfaces, and can efficiently lower the interfacial tensions and form both normal and reverse micelles in solution. Their molecular structure comprises a non-polar hydrophobic chain and a polar head of various lengths and types.1 Their composite character is described by a property known as hydrophilic lipophilic balance i.e., HLB.2 It is the HLB which primarily decides their micellization, dispersion and emulsification activities. The surfactants, detergents, soaps and lipids are amphiphilic compounds and play vital roles in the surface chemical applications, and basic research.
A large number of drug molecules are amphiphilic and self-associate in aqueous environments to form small aggregates.3 Although the pharmacological effects of amphiphilic drugs are usually manifest at concentrations well below the CMC, it is likely that accumulation of drug molecules in certain sites in the body may cause a localized high concentration resulting in aggregation and consequent changes in biological activity due to decreased transport rates or decreased ability to pass through biological barriers4. In addition amphiphilic drugs also provide an opportunity for an examination of the influence of the structure of the hydrophobe on the association characteristics of surface active molecules.
The tricyclic antidepressant drugs are widely studied interesting series of compounds showing association properties.5-8 It has been well established that aggregates of approximately 8-10 monomers of these compounds are formed in water by a closed association process at a well defined critical concentration. The effect of additives like b-cyclodextrin on the aggregation behaviour of these amphiphilic anti-depressants have also been evaluated by determining their apparent critical aggregation concentration. Recently self-assembling properties of hydantoin drug with SDS and CTAB have also been widely studied.9-10
In order to establish the aggregation behaviour of all amphiphilic drugs in aqueous solution an attempt has been made to investigate micellization tendency of some selected antibiotics. In the first attempt the aggregation behaviour of cefroprozil monohydrate has been reported after successful investigation”. The present piece of research investigation is an extension of our previous study in order to generalize the self-assembling characteristics of amphiphilic anti-biotics like CA.
In order to establish the aggregation behaviour of all amphiphilic drugs in aqueous solution an attempt has been made to investigate micellization tendency of some selected antibiotics. In the first attempt the aggregation behaviour of cefroprozil monohydrate has been reported after successful investigation”. The present piece of research investigation is an extension of our previous study in order to generalize the self-assembling characteristics of amphiphilic anti-biotics like CA.
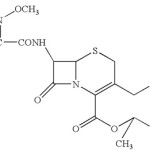 |
Scheme 1:
Cefuroxime Axetil (CA) C20H22 N4 O10S (Mol.Wt. = 510.48)
|
CA is semi –synthetic broad-spectrum cephalosporin antibiotic for oral administration. It is used in the treatment of tonsillitis, strep throat, ear infection skin infection and bronchitis. It is less susceptible to beta-lectamyse and so may have grater activity against haemophilus influenza, Neisseria gonorrhoea and lyme disease.
Material and Methods
Spectrophotometric method was employed to determine CMC of CA due to its non-conducting nature. A sharp deviation in the slope was observed in the plot of absorbance vs. concentration of CA. Variation in the CMC of CA in presence of SDS and CTAB was also determined spectrophotometrically. Variation in the CMC of SDS and CTAB in presence of CA was determined by conductivity method. Both conductivity meter and spectrophotometer are of systronics make. Double distilled water with specific conductance less than 5 ms was used in all experimental purposes. The drug cefuroxime axetil has been procured as a gift from Lupin Limited, Mandideep, Raisen (M.P.)
Result and Discussion
The compound CA absorbs in the ultraviolet region showing absorption maximum at 276 nm. Since it is non-conducting in nature spectrophotometric technique is preferred to determine its CMC. The CMC value found by this technique is 0.2 mM. Since it is readily soluble in water such aggregation at very low concentration is not unusual. The plot of absorbance vs. concentration for CA is shown in Fig. 1.
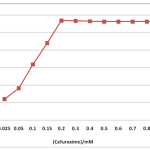 |
Figure 1: CMC of CA by Spectrophotometric method |
The contribution of anionic surfactant SDS in the micellization of CA has been studied by fixing the concentration of SDS in aqueous solutions of CA during its CMC measurement spectrophotometrically. All measurements are carried out in the premicellar region of SDS. The variation in CMC of CA in presence of SDS is reported in Table-1
Table 1: CMC of CA in presence of SDS
| Sr.No. | [SDS]/mM | CMC/mM |
| 1. | 0 | 0.2 |
| 2. | 0.05 | 0.15 |
| 3. | 1.0 | 0.14 |
| 4. | 2.5 | 0.13 |
| 5. | 5.0 | 0.12 |
The result shows a regular decrease of CMC by increasing the concentration of SDS. This is in accordance of with established trend that the aggregation of drug is favoured by the addition of surfactants from outside10-12. This may be due to structural similarity of SDS and CA. The CA has a seven membered elongated chain alongwith other cyclic part. The inclusion of sodium dodecyl sulphate moity certainly may favour the formation of aggregates of CA. Similarly participation of CTAB in the aggregation of CM has been investigated by determining CMC of CA at varying concentration of CTAB. The result is presented in Table-2
Table 2 : CMC of CA in presence of CTAB
|
Sr.No. |
[CTAB]/mM |
CMC/mM |
|
1. |
0 |
0.2 |
|
2. |
0.05 |
0.15 |
|
3. |
0.1 |
0.14 |
|
4. |
0.2 |
0.14 |
|
5. |
0.3 |
0.14 |
|
6. |
0.5 |
0.14 |
Here a regular decrease in the CMC of CTAB is observed up to 0.1 mM beyond which the CMC value remains unaltered. This may be due to the fact that micelle structure of CA has some limitations for accommodation of surfactants monomers above a particular concentration. Secondly, both CTAB and CA are of comparable size and steric effect might be playing significant role beyond a particular concentration limit. Due to steric crowding the passage of CTAB in the micellar bed of CA may be checked thereby limiting the participation of surfactant in the aggregation of drug.
The interaction between CA and surfactants like SDS and CTAB has been studied by measuring the variation in the CMC of SDS and CTAB in presence of CA. All measurements are carried out in the premicellar region of CA. The counter-ion association b have been determined for both SDS as well as CTAB-CA mixed micelles. The degree of dissociation a was first determined from the specific conductance vs. concentration of surfactant plot. Actually a is ratio of the post micellar slope to the premicellar slope of these plots. The b of the micelles is equal to 1-a. The results for both SDS-CA and CTAB-CA mixed micelles has been shown in table 3.
Table 3 : CMC and counter-ion association b of SDS and CTAB in presence of CA
| Sr. No. | [CA]/mM | CMC/mM for SDS | b for SDS micelles | CMC/mM for CTAB | b for CTAB micelles |
| 1. | 0 | 7.8 | 0.67 | 0.86 | 0.25 |
| 2. | 0.02 | – | – | 0.5 | 0.215 |
| 3. | 0.04 | 4.0 | 0.75 | 0.4 | 0.417 |
| 4. | 0.06 | 3.7 | 0.5 | 0.35 | 0.231 |
| 5. | 0.08 | 3.4 | 0.66 | 0.3 | 0.474 |
| 6. | 0.1 | 2.5 | 0.58 | 0.27 | 0.385 |
The CMC of SDS as well as CTAB regularly decrease on gradual increase in the concentration of CA. It follows the established trend and reflects the proper formation of SDS – CA and CTAB – CA mixed micelles. The mixed micell formation is driven due to higher hydrophobicity of CA and longer surfactant like chain in its structure. However, in both the cases the low concentration of CA seems to be more effective for mixed micelle formation. The b values for both mixed micellar systems are not indicative of any specific information. This might be due to complex interactions in such mixed micellar aggregates.
Physicochemical parameters like free energy of micellization DG–m , enthalpy of micellization DHom and entropy of micellization DGom have been calculated utilizing biphasic model for both SDS-CA and CTAB-CA mixed micellar systems at three temperature 30oC, 40oC and 50oC. DHom has been estimated from the slope of the plot of ln(CMC/288.4/55.6) vs. for SDS – CA mixed micelles and from the plot of ln(CMC/364.5/55.6) vs. for CTAB – CA mixed micelle system. Here pure surfactant has been taken as the reference state13. DGom has been calculated by the relation DGom = RT ln (CMC / 288.4 / 55.6) for SDS – CA system and DGom = RT in (CMC/364.5/55.6) for CTAB – CA mixed micellar system. DSom has been determined by the well known thermo-dynamic relation DGom = DHom – TDSom for both SDS – CA and CTAB – CA mixed micellar systems. The parameters calculated are shown in Table-4.
Table-4 : Thermodynamic parameters of SDS – CA and CTAB – CA mixed micellar systems.
|
Temp. |
CMC/ mM |
DGom (KJ/mol) |
DHom (KJ/mol) |
DSom (JK-1mol-1) |
| A) SDS – 0.04 mM303313323B) SDS-0.06 mMCA
303 313 323 A) CTAB – 0.02mM CA 303 313 323 B) CTAB-0.04mM CA 303 313 323 C) CTAB = 0.06 mM CA 303 313 323 |
4.0 4.7 6.2 3.7 4.2 5.2 0.5 0.6 0.6 0.4 0.6 0.7 0.35 0.6 0.7 |
-38.29 -39.14 -39.75 -38.49 -39.42 -40.12 -44.08 -45.02 -46.19 -44.68 -45.10 -45.97 -44.84 -45.09 -46.13 |
-2.0 -1.5 -1.2 -2.8 -3.0 |
+119.8 +118.6 +116.9 +122.0 +121.1 +119.5 +141.5 +140.0 +139.3 +138.2 +135.0 +133.6 +138.0 +134.5 +133.5 |
It is evident from the above data that formation of mixed micelle is favoured by negative values of free energy in all cases. However, high positive value of entropy of micellization serve as driving force and main cause for mixed micelle formation.
Conclusion
Cefuroxime axetil forms micellar aggregates above a particular concentration range. Its aggregation is supported by surfactants like SDS and CTAB. However, in the bed of drug aggregates CTAB monomers have accommodation limit above certain concentration range. The micelle formation of SDS and CTAB is also assisted in the presence of CA in its premicellar region. The variation in CMC of SDS and CTAB in presence of CA is following established regular trend. Thermodynamic parameters are favorable for mixed micelle formation between SDS – CA and CTAB – CA
Acknowledgements
Authors are thankful to Principal, RIE, Bhopal for providing laboratory facilities. Lupin Limited Mandideep is thankfully acknowledged for providing pure sample of CA.
References
- Myer, D., Surfactant Science & Technology, VCH/Pub. INC, 1998
- Sharman, P., Emulsion Science, Academic Press London, 1968.
- Taboda, P., Attwood, P., Ruso, M.J., Garcia, M. and Mosquera, V., Phys. Chem. Chem. Phys., 2, 5175 (2000)
- Attwood, D. and Florence, A.T., Surfactant Systems, Chapman and Hall, London, 1983.
- Taboda, P., Russo, J.M., Garcia, M. and Mosquera, V. Collid Polym. Sci. 281, 575 (2003).
- Harned, H.S. & Owen, B.B., Physical Chemistry of Electrolyte Solutions, Chapman and Hall, London, 1975 (Chapter 8)
- Junguera, E., Romera, J.C., and Aicart, E., Langmuir 17, 1826 (2001).
- San Andres, M.P., Sicilia, D., Rubio, S. and Perez-Bevdito, D., J. Pharm. Sc., 87 (7), 821 (1998).
- Krishan, R.S.G., Thennarasu, S. and Mandal, A.B., Chemical, Physics, 291, 195 (2003).
- Tiwary, L.K., Mandal, A; Alam, M.S., Thennarasu S. and Mandal, A.B., Colloid and Surfaces B, 82, 126 (2011).
- Sharma, P. and Tiwary, L.K., oriental J. Chem., 26 (4), 1491 (2010).
- James, J. Vallaichami, S., Krishnan, R.S.G., Samikannu, S. and Mandal, A.B., Chemical Physics, 312, 275 (2005).
- Chatterjee, A., Moulik, S.P. Sanyal, S.K., Mishra, B.K. and Puri, P.M, J. Phys. Chem. B, 105, 12823 (2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.









