Extraction of Polyphenol, Flavonoid from Emblica Officinalis, Citrus Limon, Cucumis Sativus and Evaluation of their Antioxidant Activity
Madhu Agarwal¹*, Arvind Kumar¹, Ragini Gupta² and Sushant Upadhyaya¹
¹Department of Chemical Engineering, MNIT, Jaipur (India). ²Department of Chemistry, MNIT, Jaipur (India).
The antioxidant potential of three commonly used edible fruits and vegetables Emblica officinalis (Amla), Citrus limon (lemon) and Cucumis sativus (cucumber) available in local market of Jaipur (India), was estimated. The parts of food material taken for this study is edible part of Emblica officinalis, peel of Citrus limon and Cucumis sativus. The total phenolic content, antoxidant activity and flavonoid content was estimated by folin-ciocalteau method, phosphomolybdenum assay and aluminium chloride colorimetric technique. All the three activities determined were found maximum in Emblica officinalis, but Citrus limon and Cucumis sativus also show good amount of these activities. Thus the study revealed that the peel of Citrus limon and Cucumis sativus (otherwise waste) can also be used as a good source of antioxidant activity.
KEYWORDS:Antioxidant; Polyphenol; Flavonoid; Amla; Lemon Peel; Cucumber Peel
Download this article as:| Copy the following to cite this article: Agarwal M, Kumar A, Gupta R, Upadhyaya S. Extraction of Polyphenol, Flavonoid from Emblica Officinalis, Citrus Limon, Cucumis Sativus and Evaluation of their Antioxidant Activity. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Agarwal M, Kumar A, Gupta R, Upadhyaya S. Extraction of Polyphenol, Flavonoid from Emblica Officinalis, Citrus Limon, Cucumis Sativus and Evaluation of their Antioxidant Activity. Available from: http://www.orientjchem.org/?p=23515 |
Introduction
The biological antioxidants are compounds that protects biological systems against the potentially harmful effects of processes or reactions that causes excessive oxidation. Nowadays the oxidative stress is one of the serious problems of the modern society. Different air pollutions, smoking, UV radiation etc. leads to oxidative stress of cells, causes various diseases like dermatitis, melanomas or photo aging of the skin, cancer, heart disease, inflammation, arthritis, immune systems decline, brain dysfunction and cataracts etc. The consumption of fruits and vegetables has been found to be associated with lowering of these diseases as they contain a large amount of phenolic compounds, antioxidants and flavonoids. In various studies it has been found that antioxidants can inhibit or delay the oxidation of an oxidisable substrate in a chain reaction hence antioxidants seem to be very important in prevention of these diseases (Ali et al. 2010; Ames 1983; Ames et al. 1993; Feskanich et al. 2000; Halliwell 1996). The extraction of phenolic compounds can be defined as the separation of medicinally active portion from plant tissues using selective solvents through standard extraction procedures. The main criteria of extraction technique is separating the soluble and insoluble components and leaving behind only insoluble Cellular mass. The extraction products of plants have relatively complex mixtures covering a number of groups of plant metabolites either in liquid form or semi-solid state (Imran et al. 2011; Ramamoorthy et al. 2007). The general techniques of extraction of medicinal plants include maceration, infusion, percolation, digestion, decoction, hot continuous extraction (soxhlet), aqueous-alcoholic extraction by fermentation, counter current extraction, microwave assisted extraction, ultrasound extraction (sonication), supercritical fluid extraction (SFE), phytonic extraction (with hydro-flouro-carbon solvents), etc. For the aromatic plants, three types of hydro-distillation techniques (water distillation, steam distillation, steam and water distillation), hydrolytic maceration followed by distillation technique, expression method and enfleurage method (cold fat extraction) can be used. Some of the latest methods of extraction for aromatic plants include head space trapping technique, solid phase micro-extraction, protoplast extraction technique, micro-distillation, thermo-micro-distillation, and molecular distillation techniques (Ics-Unido 2006).
The aim of this study is to find out the potential of fruit & vegetables and their waste in terms of their total phenolic, flavonoid content & antioxidant activity. In order to determine these activities a comprehensive study was carried out for 3 plant species collected from local market of Jaipur. The fruits selected were Emblica officinalis, Citrus limon and Cucumis sativus.
Materials and Methods
Materials
Three different types of fruits & vegetables were purchased from local market of Jaipur, Rajasthan (India). These include Amla (Emblica officinalis), Lemon (Citrus limon) and Cucumber (Cucumis sativus). For the present study the parts of fruit taken are, edible part of amla, peel of lemon and peel of cucumber. Folin-Chiocalteu reagent, Sodium carbonate, Gallic acid, Quercetin, Methanol, Aluminium chloride, Potassium acetate, Butylated Hydroxy Toluene (BHT), Sulphuric acid, Sodium phosphate, Ammonium molybdate of AR grade were purchased from local supplier of Jaipur.
Sample preparation
All the fruits, vegetables taken were washed with distilled water and desired parts were dried in oven at 70 oC. After that the parts were grinded and sieved with mesh aperture size of 2 mm. The portion of fruits (1gm) was homogenized with different concentrations of aqueous methanol solution (0-70% methanol). The homogenate was stir with magnetic stirrer at 900 rpm at room temperature for 30 minutes. The extract was centrifuged at 3000 rpm for 20 minutes. The supernatant was removed and filtered with whatmann filter paper 4. Finally the supernatant was stored in the dark. Similarly another extraction was done keeping the methanol: water (50:50 v/v) ratio constant but varying the amount of sample dose (1gm, 1.5 gm, 2.0gm, 2.5gm).
Determination of total phenolic content
The total phenolic content of the fruit and vegetable extracts were estimated by the method described by Singleton and Rossi (1965) with slight modification and reported as Gallic acid equivalent (GAE) per gram of fruit/vegetable. Gallic acid in various concentrations (20-140
Determination of antioxidant activity
The antioxidant activity of fruit and vegetable extracts were estimated by phosphomolybdenum assay and reported as Butylated Hydroxy Toluene equivalent (BHTe) per gram of fruit/vegetable. Different concentrations of Butylated Hydroxy Toluene (25-125 μg/ml) in distilled water were prepared as standard. 0.3 ml of fruit extracts were taken in test tubes. The reagent solution was prepared by mixing 10 ml of 0.6 M sulphuric acid, 10 ml of 28 mM sodium phosphate and 10 ml of 4 mM ammonium molybdate into a beaker and 3 ml reagent solution was added to all the tubes. 0.3 ml of methanol served as blank. All the tubes were incubated at 95 oC for 90 min. The tubes were cooled to room temperature and the optical density was measured at 695 nm using UV spectrophotometer (UV-1800,Shimadzu).
Determination of total flavonoid content
The total flavonoid content of the fruit and vegetable extracts were estimated by using aluminium chloride colorimetric technique and reported as Quercetin equivalents (Qe) per gram of fruit/vegetable. Quercetin in various concentrations (12.5-100 μg/ml) was prepared in methanol as standard. 0.5 ml of each methanol extracts (1:10) were taken in test tubes and 1.5 ml methanol, 0.1ml of 10% aluminium chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml distilled water were added separately to each tubes. All the tubes were incubated at room temperature for 30 min optical density was measured at 415 nm by using UV spectrophotometer (UV-1800,Shimadzu).
Results and Discussion
The total polyphenol content, antioxidant activity and flavonoid content was determined by folin-ciocalteau method, phosphomolybdenum assay and aluminium chloride colorimetric technique respectively as stated above.
Total Polyphenol content
The standard curve (y = 0.012 x, r2 = 0.986) for determination of total polyphenol content was obtained by measuring OD of standard solution of Gallic acid and is shown in Figure 1. The Total phenolic content of all the three samples/extracts were then estimated using this standard curve and results were shown in Figure 2.
Effect of solvent Concentration
It can be seen from the Figure 2.a that polyphenol content of Emblica officinalis, extract increases with increase in concentration of methanol in the solvent. The maximum amount of polyphenol extracted was 112.52 mg GAE/g at 70% methanol in water nearly equal to amount (111.72 mg GAE/g) obtained at 50% methanol in water. Similarly Citrus limon (peel) and Cucumis sativus (peel) also shows an increase in total polyphenol content with increase in concentratuion of methanol but the increase is not very high after 50% methanol concentration. The maximum amount of polyphenol extracted was 9.7 and 7.4 mg GAE/g for lemon peel and cucumber peel respectively at 70% methanol in water. Therefore, 50% methanol in water was taken as optimum solvent concentration for further study.
Effect of amount of dry sample powder
The 50% methanol solution was used to extract total polyphenol from all the three samples by varying the amount (1-2.5 g) of dry powder of fruit/vegetable, the results obtained were shown in Figure 2.b. It can be seen from the figure that total phenolic content of extract increases with increase in amount of dry sample. For 2.5 g of sample it is 194, 16.072, 10.0548 mg GAE for amla, lemom and cucumber peel respectively. It can be seen that Emblica officinalis shows maximum phenolic content as actual fruit part is taken for study. Both Citrus limon and Cucumis sativus shows comparable amount of phenolic content as waste part was taken for the study (peel). Though amla contains very good amount of phenolic compounds, the amount of phenolic compounds in lemon and cucumber peels are good enough to extract. Therefore these materials thrown away everyday from households as waste can be used for extraction of valuable phenolic compounds.
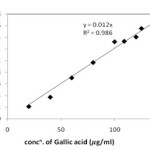 |
Figure 1 Standard curve for determination of Total phenolic content |
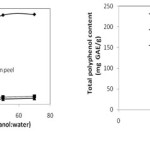 |
Figure 2: (a) Effect of solvent concentration (b) Effect of amount of sample on extraction of total polyphenolics compounds |
Total Flavonoid content
The standard curve (y = 0.005 x, r2 = 0.994) for determination of total flavonoid content was obtained by Aluminium chloride colorimetric technique as Quercetin equivalents and is shown in Figure 3. The Total flavonoid content of all the three samples/extracts were then estimated using this standard curve and results were shown in Figure 4.
Effect of solvent Concentration
It can be seen from the Figure 4.a that total flavonoid content of all the three sample extract increases with increase in concentration of methanol in the solvent but the increase is not very sharp. The maximum amount of flavonoid extracted was 19.2,18.1,17.1 mg Qe/g of amla, lemon peel, & cucumber peel respectively, at 70% methanol in water nearly equal to amount (111.72 mg GAE/g) obtained at 50% methanol in water. Therefore, 50% methanol in water was taken as optimum solvent concentration for further study. Diankov et al. (2011) also found that solvent concentration does not affect significantly the extraction rate. It can also be seen from the figure that similar trend was obtained all the three samples with respect to solvent concentration and the amount of flavonoid content is also comparable.
Effect of amount of dry sample powder
The 50% methanol solution was used to extract total flavonoid from all the three samples by varying the amount (1-2.5 g) of dry powder of fruit/vegetable, the results obtained were shown in Figure 4.b. It can be seen from the figure that total flavonoid content of extract increases with increase in amount of dry sample. For 2.5 g of sample it is 22.41, 21.34, 20.28 mg Qe for amla, lemom and cucumber peel respectively. It can be seen that in terms of total flavonoid content all the three samples are comparable. Though amla contains higher amount of flavonoids, the amount present in lemon and cucumber peels is nearly equal to it. Therefore, these waste materials can be used for extraction of valuable flavonoids instead of costly amla fruit.
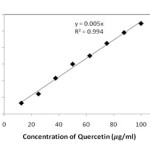 |
Figure 3: Standard curve for determination of Total flavonoid content |
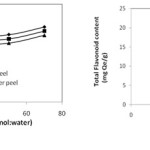 |
Figure 4: (a) Effect of solvent concentration (b) Effect of amount of sample on extraction of total flavonoids |
Antioxidant activity
For determination of total antioxidant activity Phospho molybdenum assay and metal chelating activity are reported as Butylated Hydroxyl Toluene (BHT) equivalents. The standard curve (y = 0.013 x, r2 = 0.992) obtained is shown in Figure 5. The antioxidant activity of all the three samples/extracts was estimated using standard curve and results were shown in Figure 6.
Effect of solvent Concentration
It can be seen from the Figure 6.a that antioxidant activity of all the three sample extract increases with increase in concentration of methanol in the solvent but the increase is not very sharp. The maximum antioxidant activity shown was 8.54, 2.91& 1.75 mg BHTe/g of amla, lemon peel, & cucumber peels respectively, at 70% methanol in water. It can also be seen from the figure that maximum antioxidant activity was shown by amla. Lemon and cucumber pees also show the antioxidant activity therefore these can be used as cheap source of antioxidants.
Effect of amount of dry sample powder
All the three samples were analyzed for their antioxidant activity by varying the amount (1-2.5 g) of dry powder of fruit/vegetable; the results obtained were shown in Figure 6.b. It can be seen from the figure that total antioxidant activity of extract increases with increase in amount of dry sample. For 2.5 g of sample it is 13.132, 3.332, 2.352 mg of BHT/ 2.5 g for amla, lemom and cucumber peel respectively. It can be seen that in terms of antioxidant activity amla is superior to lemon and cucumber peel. Though amla shows higher antioxidant activity, good activity was also shown by lemon and cucumber peels.
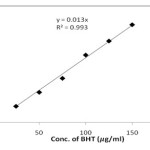 |
Figure 5: Standard curve for determination of Antioxidant activity |
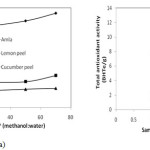 |
Figure 6: (a) Effect of solvent concentration (b) Effect of amount of sample on antioxidant activity
|
Conclusions
In the present study, 50% aqueous solvent extracts from three fruit and vegetable sample gave higher amounts of total polyphenol and antioxidant activity. It can be concluded that polyphenol content is proportional to antioxidant property of samle as amla having higher polyphenol content shows higher antioxidant property compared to lemon and cucumber peels. It can also be concluded that there is no direct relationship between total phenolic compounds present in the sample and the total flavonoid content. Amla having very high amount of total phenolic content shows similar flavonoid content with lemon and cucumber peels. Thus lemon and cucumber peels can be considered as cheap source of flavonoid, and can be used efficiently for its extraction. These materials, otherwise waste can be used as potential source of antioxidants for industrial application also.
References
- Ali M. A., Devi L. I., Nayan V., Chanu Kh. V., Ralte L., International Journal of Biological & Pharmaceutical Research,1,76(2010).
- Ames B. N., Science, 221,1256(1983).
- Ames B. N., Shigenaga M. K., & Hagwn T. M., Proceedings of the National Academy of Sciences, USA, 90,7915(1965).
- Feskanich D., Zeigler R. G., Michaud D. S., Giovannucci F. L., Speizer F. E., Willett W. C. & Colditz W. C., Journal of the National Cancer Institute, 92,1812(2000).
- Halliwell B., Annual Review of Nutrition, 16, 33(1996).
- Imran M. M., Raja M. M. M., Basith J. A., and Asarudeen A., International Food Research Journal, 18,579(2011).
- Ramamoorthy P. K., Bono A., Journal of Engineering Science and Technology, 2,70(2007).
- Ics-Unido, South-East Asian (SEA) regional workshop on “extraction technologies for medicinal and aromatic plants”, International Centre for Science and High Technology (2006).
- Singleton V. L. & Rossi J. A., American Journal of Enology and Viticulture, 16, 144(1965).
- Diankov S., Karsheva, M., Hinkov I., Journal of the University of Chemical Technology and Metallurgy,46, 315(2011).

This work is licensed under a Creative Commons Attribution 4.0 International License.









