Synthesis of Spermidine using Solidphase Synthetic Strategy
V. Santhakumari
Sree Narayana College, Kollam, Kerala, India.
Spermidine is a naturally occurring polyamine which regulate growth processes in living beings. Using solid phase synthetic strategy spermidine was synthesised. The only purification process involved throughout the synthesis is filtration and washing with suitable solvents. Spermidine was first synthesised over polystyrene support and then cleaved from matrix photolytically. The yield and purity of the sample was studied using differently cross linked polystyrene resin with DVB as the cross linking agent. The effect of one photocleavable linker was studied. The synthesis of linker was done by classical organic synthetic procedure.
KEYWORDS:Solid phase organic synthesis; Spermidine; linkers; photolysis; functionalisation
Download this article as:| Copy the following to cite this article: Santhakumari V. Synthesis of Spermidine using Solidphase Synthetic Strategy. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Santhakumari V. Synthesis of Spermidine using Solidphase Synthetic Strategy. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23984 |
Introduction
Solid phase synthesis is a methodology whereby synthetic transformations are conducted with one of the reactant molecules attached to an insoluble material referred to as the solid support. It was originally developed for peptide synthesis. Since the recent impact of combinatorial chemistry, solid phase techniques have been applied more generally to organic synthesis. One of the requirements of solid phase chemistry is a linker to attach a substrate molecule to the solid phase. As synthesis proceeds, this material is transformed to the product, which can be finally removed by cleavage of the linker.
All polyamines especially Spermidine is intimately involved in normal growth processess 1,2,3 Spermidine and their conjugates play an important role in the proliferations , differentiation and growth cycles of prokaryotic and eukaryotic4 cells. Polyamine levels in cancer cells are much higher than in normal cells and hence polyamine levels are exploited in chemotherapy5,6,7
The present work of synthesis of Spermidine involves the synthesis of 1%, 2% and 4% crosslinked polystyrene matrix using DVB as the crosslinking agent. They are functionalized to get chloromethylated polystyrene resin (Merrifield resin). Merrifield resin was first nitrated and then subjected to Gabriel’s phthalimide synthesis to get 3-nitro-4-aminomethyl polystyrene . Using this resins, synthesis of Spermidine was done.
3-nitro-4-aminomethyl polystyrene is an o-nitrobenzyl linker which is photocleavable which was attached to differently crosslinked polystyrene matrix and treated with appropriate reagents to obtain polystyrene supported Spermidine. Finally, Spermidine was cleaved from the support by photolysis.
Chemistry
Synthesis of DVB Crosslinked Polystyrene with Variable Degree of Crosslinking (A)
Polystyrene- divinyl benzene (P.S.-DVB) copolymer supports of different degrees of crosslinking were prepared. The monomers, styrene and DVB were made free from inhibitors and were used for polymerisation. Calculated amounts of the monomers mixed with the initiator, benzoyl peroxide were added to water, kept stirring at 80oC containing poly (vinyl pyrrolidine) as the suspension stabilizing agent. The details of the reaction are presented in Table I.1 & reaction sequence is presented in Scheme I.
 |
Scheme 1 |
Preparation of chloromethylated polystyrene (B)
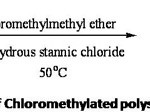
Anhydrous zinc chloride and stannic chloride have been used in the chloromethylation of crosslinked polymers.8 . For chloromethylation, the adopted procedure9 was to add polystyrene beads (A), preswollen in dichloromethane to a well stirred suspension of anhydrous stannic chloride in chloromethyl methyl ether kept at 0oC. Polystyrene resin which was allowed to preswell in a good organic solvent was normally used since it was found to give better reaction efficiency. The product resin was dried under vacuum at 60oC. The reaction is depicted in Scheme II. The chlorine capacity was determined using modified Volhard’s . The chlorine capacity is tabulated in Table II.
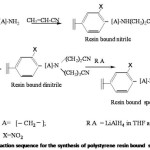 |
Scheme 2 |
Preparation of Photocleavable Linker
Photolysis offers a mild and potentially orthogonal method of cleavage that takes place under neutral conditions. Photocleavable protecting groups have been used widely in carbohydrate chemistry, nucleotide and peptide synthesis.
Preparation of 3-nitro-4-aminomethyl Polystyrene (I)
The first step in the preparation of 3-nitro-4-aminomethyl Polystyrene (I) is the preparation of 3-nitro 4-chloromethyl polystyrene (C). Polystyrene could be nitrated easily using nitric acid.10 It has been found to be a low temperature reaction and the optimum temperature was found to be 0- 5oC. Fuming nitric acid of specific gravity 1.42 is enough and mono nitration occurs as was evident from the percentage of nitrogen content. The presence of nitro group was supported by elemental analysis. The presence of nitro group at 3-position of the phenyl ring was supported by I.R. spectra which showed strong absorption bands at 1525 cm-1 and 1350 cm-1.Analytical and spectral details are given in Table II. The reaction is given in Scheme: III.
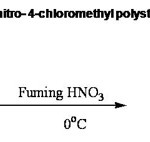 |
Scheme 3 |
3-nitro-4-aminomethyl polystyrene was prepared from 3-nitro-4-chloromethyl polystyrene by Gabriel’s phthalimide synthesis11 which involves two steps:- 3-nitro-4-chloromethyl polystyrene, pre-swollen in DMF was reacted with potassium phthalimide. The phthalimidomethyl resin formed showed IR absorption due to C-N str. at 1380 cm-1 and C=O str. at 1740 cm-1. Phthalimidomethyl resin was subjected to hydrazinolysis. Resin in ethyl alcohol was mixed with hydrazine hydrate and heated under reflux. The presence of free NH2 group was confirmed by ninhydrin test. (Scheme IV.). The amino capacity of the resin was determined by titrimetric method. Spectral and analytical data are given in Table II.
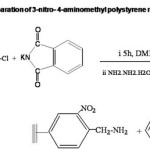 |
Scheme 4 |
Table 1: Yield and crosslink density of DVB crosslinked polystyrene
|
Crosslink density mole % |
Volume of Monomers |
% yield |
|
| Styrene (mL) | DVB (mL) | ||
|
1 2 4 |
18.7 18.7 18.7 |
0.45 0.9 1.8 |
88 90 91 |
Table 2: Spectral and Analytical details of chloromethylated polystyrene resin and aminomethyl polystyrene resin
|
Crosslink density Mole % |
Chlorine capacity mmoles/g. |
NH2 Capacity mmoles /g |
IR (KBr) cm-1 |
|
|
Resin-C |
Resin-I |
|||
|
1 2 4 |
2.48 2.18 1.92 |
2.21 1.98 1.80 |
1350 cm–1, 1525 cm-1 (NO2str) |
3520 cm-1 (NHstr )
|
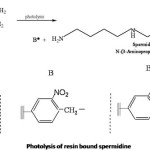
Preparation of resin bound spermidine
Differently crosslinked polystyrene resin bearing photocleavable linker was reacted with acrylonitrile in THF12 to get the mononitrile which give C N str. frequency at 2200 cm-1. The resin bound mononitrile was heated with chlorobutyronitrile to get the dinitrile.13 The dinitrile was reduced with appropriate reducing agents to get the resin bound spermidine. After reduction the resin gave ninhydrin test and IR spectrum showed NH str at 3520 cm-1 and 3480 cm-1 . The general reaction sequence is given by Scheme V.
 |
Scheme 5 |
The reduction of the monoalkylated product was done selectively to avoid the reduction of the chromophoric group present in the linkers. Chromophoric groups are introduced into the linker moiety for avoiding the destruction of the polyamine. The chromophoric group sel-ectively absorbs light and cleavage of synthetic polyamine from the support occurs. The reduction of resin I and was done with lithiumaluminium hydride in THF at 50oC.
Photolysis of polystyrene bound Spermidine
Photolysis offers a mild and potentially orthogonal method of cleavage that takes place under neutral conditions for the release of the synthesised product from the solid support. Synthesis was carried out effectively on solid supports in earlier days, but the cleavage was found to be difficult one. The smooth release of the product can be achieved by using photocleavable linkers. In the case o-nitrobenzyl protecting groups, the nitro group is reduced to a nitroso function while one oxygen atom get inserted into the C-H bond located in the ortho position. The reaction appears to be general for all ortho substituted nitrobenzene derivatives having α hydrogen atom in the side chain.
Spermidine was synthesised stepwise on an o-nitrobenzyl resin attached to polystyrene resin. The differently crosslinked polystyrene resin bound spermidine was subjected to photolysis in a RPR 100 apparatus equipped with immersion type photometer. Wavelength below 320nm was filtered out to avoid unwanted side reactions. Photolysis was continued for 12-36h.
The linker is photocleavable which release Spermidine without decomposing them and leaving behind the aldehyde polymer which remains insoluble and can be filtered out.
Photolysis of differently crosslinked polystyrene bound spermidine
Using differently crosslinked resin bound spermidine photolysis was carried out by suspending the resin in methanol. After irradiation the aldehyde polymer was filtered out and purified by chromatographic separation. The general scheme of photolysis of polystyrene bound spermidine resin is given in Scheme:VI, In the case of spermidine, homospermidine and thermospermine the authentic data are available for its hydrochloride salt. Hence, they are converted as hydrochloride salt by adding 6N HCl and precipitated by adding 1:1 ethanol-methanol mixture. The yield of spermidine is presented on Table III. The spectral and analytical data of spermidine is presented on Table .IV.
Table 3: Effect of extent of cross linking and yield of spermidine
 |
Table 1: Click here to View table |
| % Yield of Spermidine | ||
| 1% | 2% | 4% |
| 61.20 | 63.1 | 60.01 |
Table 4: Spectral and analytical details of spermidine hydrochloride
|
Elemental Analysis (%) |
IR (KBr) | 1HNMR | 13CNMR | ||
|
Element |
Calculated |
Found |
|||
| C
H
N
Cl |
33.54
8.46
16.53
41.71 |
33.01
8.64
16.50
41.85 |
NHstr,3400cm-1 |
δ 3.45 (t,2H)
δ 3.35 (m,2H) δ 2.93 (m,4H) δ 1.93 (m,2H) δ 1.64 (m,4H) |
4 peaks
δ 31 (2C) δ 28 (4C) δ 23 (2C) δ 22 (4C)
|
Photolysis of differently crosslinked polystyrene bound Spermidine
Polystyrene resin bound Spermidine (5g) suspended in methanol (200 mL) was irradiated for 15h in an RPR-100 apparatus equipped with immersion type photo reactor with wavelength 340 nm which released Spermidine and nitroso aldehyde polymer. The wave length below 320 nm was filtered out by circulating 40% CuSO4 solution through the outer jacket. The nitroso aldehyde polymer was filtered out and its IR spectrum showed C=O str frequency at 1700 cm-1. The filtrate was collected quantitatively and purified by column chromatography. Filtrate was concentrated by evaporation and made to a paste by adsorbing on neutral alumina and eluted using ethyl acetate as the eluent. Distillation under vacuum gave a colourless liquid which was subjected to analysis. The spectral and analytical data were compared with that of authentic sample.
Experimental
The monomers, styrene and DVB were obtained from Sigma Aldrich Corporation, USA and were distilled under reduced pressure. All other chemicals of AR grade were procured from local vendors. All solvents were purified by standard procedures. The IR spectra were recorded on a Perkin Elmer spectrum I spectrophotometer using KBr pellets at MaduraiKamarajUniversity. The NMR spectra were recorded on a Joel GSX 400 MHz FTNMR spectrometer at SAIF, IIT, Chennai. CHN analysis was done at RRL, Thiruvananthapuram.
Preparation of polystyrene –DVB support
The monomers, styrene and DVB were purified by low-pressure distillation. They were washed with sodium hydroxide (1% solution) three times and water to remove inhibitors. The crosslinked polymers were prepared by suspension polymerisation technique. Poly (vinyl pyrrolidine) (150 mg) was dissolved in distilled water (100 mL) and heated to 80oC. The mixture of monomers, styrene (18.7 mL) and divinyl benzene (0.9 mL), for 2% degree of crosslinking, was mixed with the initiator benzoyl peroxide (200 mg) and added slowly to the hot solution with constant stirring. The mixture was heated at 90oC with continuous stirring for 12 h. The polymer formed during the process was collected by filtration and washed with hot water, ethanol, toluene, acetone and methanol and the beads were dried at 60oC in vacuum. Polymers with different degree of crosslinking, viz. 1%, and 4% were prepared by varying the ratio of the monomers suitably.
Preparation of chloromethylated polystyrene
2% divinylbenzene crosslinked polystyrene (25 g) was swollen by stirring at 25oC for 1h in chloroform (150 mL) and cooled to 0oC. A cold solution of anhydrous SnCl4 (3.8 mL) in chloromethylmethyl ether (50 mL) was added and stirred for 5h at 0oC.The mixture was filtered and washed with (1 x 10 mL) of 3:1 dioxane- water, and then with (1 x10 mL) of 3:1 dioxane -3N.HCl. The cream coloured beads were washed further with solvents which changed gradually from water to pure dioxane and then progressively to 100% methanol. Abrupt changes of solvent compositions were avoided. The chlorine capacity of the product was determined which showed 2.18 m moles of Cl / g of polymer. Yield 30 g
Preparation of 3-nitro -4-chloromethyl polystyrene
A sample of dry chloromethyl polymer ( 5g, 2.18 m moles of Cl /g ) was added slowly with stirring to 500 mL of fuming nitric acid (90% HNO3, sp. gr. 1.5) which was cooled to 0oC. The mixture was stirred for 1h at 0oC and poured into crushed ice. The beads were filtered and washed with water, dioxane and methanol and dried .The nitrogen content was found to be 6.38 m moles/g by Kjeldahl method. This was equivalent to 1.03 nitro group per aromatic ring.Yield 6.6 g
Estimation of chlorine capacity of chloromethylated polystyrene – Volhard’s method
Chloromethylated polystyrene (0.3 g) of polymer was heated in pyridine (3 mL) at 100oC for 2h. This suspension was transferred to 250 mL conical flask containing 50% acetic acid (30 mL). Conc. HNO3 (5 mL) was added, followed by slow addition of AgNO3 (10 mL, 0.1 N), during magnetic stirring. The suspension was mixed well. The excess of AgNO3 was back titrated with standard ammonium thiocynate solution using ferric alum as indicator. A blank was also conducted. From the titre values the chlorine capacity of the polymer was determined.
Estimation of amino capacity of the differently crosslinked aminomethyl polystyrene resin
Hydrochloric acid (0.1 N., 40 mL) was added to polymer amine (200 mg). It was kept for 24h. The resin was filtered , washed and the filtrate was titrated against NaOH using phenolphthalein as indicator. A blank was conducted with 0.1N. HCl and NaOH. From the titre values amino capacity was determined and was found to be 1.98 m moles / g
Preparation of 3-nitro-4-aminomethyl polystyrene (I)
The preparation of 3-nitro-4-aminomethyl polystyrene involved two steps:- preparation of the phthalimidomethyl resin and the subsequent hydrazinolysis. 3-nitro-4-chloromethyl polystyrene containing 2.18 m moles of chlorine per g was made use of. The resin (10 g) swollen in N,N` Dimethyl formamide (100 mL) was mixed with potassium phthalimide (8.9 g) and was stirred for 6 h at 100oC. Potassium phthalimide was prepared by grinding anhydrous potassium carbonate (10 g) and phthalimide (19 g) using glass mortar. Phthalimidomethyl resin was washed with distilled water (10 mL x 3) and methanol (10 mL x 3). Phthalimidomethyl resin was mixed with hydrazine hydrate (1 mL /g of the polymer) and ethanol ( 50 mL). It was heated under reflux for 6 h. The product was filtered and washed with water (10 mL), Methanol (30 mL) and finally with dichloromethane (10 mL). Yield 8.82 g.
Preparation of differently crosslinked polystyrene resin bound spermidine
Preparation of 3-nitro-4-aminomethyl polystyrene bound spermidine was a two step process in which the first step was the formation of the nitrile, the second step was the formation of the dinitrile and the third step was the reduction of the nitrile to resin bound amine. The Polymer (10 g. 2.2 m moles of NH2 / g of polymer) preswollen in THF, acrylonitrile (1.166 g, 22m moles ) was added dropwise and stirred for 12h in water bath at 30oC fitted with reflux condenser and guard tube. The product was kept for 2 h, filtered and washed successively with THF ether, ether-water and then dried. The IR spectrum of the resin showed C N str. frequency at 2200 cm-1 confirming the presence of C N group. The nitrile(10 g) was treated with chloro butyronitrile (1.14 g) in butanol (100 mL) in the presence of anhydrous sodium carbonate (3.37 g) and KI (3.71 g). The mixture was kept for 2h and stirred for 12h at 40oC in water bath with reflux condenser and guard tube. It was allowed to stand for 6h and the product was filtered and washed successively with THF ether-water, ether and finally with ethyl alcohol. Lithium aluminium hydride (0.083 g, 2.2 m moles) in anhydrous ether (10 mL) was added to resin bound mononitrile (10 g, 2.21m moles of NH2 / g) in THF (10 mL). It was kept under reflux at 50oC for 8 h. using reflux condenser and guard tube. Excess lithiumaluminium hydride was removed by adding 30% sodium hydroxide solution ( 50 mL). The product was washed with ether, ether-water mixture to obtain the resin bound spermidine. The presence of free NH2 group was confirmed by ninhydrin test. The IR spectrum showed NH str. frequency at 3520 cm-1.
Photolysis of differently crosslinked polystyrene bound Spermidine
Polystyrene resin bound Spermidie (5g) suspended in methanol (200 mL) was irradiated for 15h in an RPR-100 apparatus equipped with immersion type photo reactor with wavelength 340 nm which released putrescine and nitroso aldehyde polymer. The wave length below 320 nm was filtered out by circulating 40% CuSO4 solution through the outer jacket. The nitroso aldehyde polymer was filtered out and its IR spectrum showed C=O str frequency at 1700 cm-1. The filtrate was collected quantitatively and purified by column
chromatography. Filtrate was concentrated by evaporation and made to a paste by adsorbing on neutral alumina and eluted using ethyl acetate as the eluent. Distillation under vacuum gave a colourless liquid which was subjected to analysis.
Results and Discussion
The use of polymer matrices as supports in organic synthesis continues to be an active field in solid phase synthesis since the pioneering work of R.B. Merrifield in the synthesis of a tetrapeptide for the first time using polystyrene support. Polymer supports and polymer supported reagents have become versatile because of its advantages like stability, easy work-up and selectivity.
A large number of new polymer supports were developed from polystyrene which were obtained by the polymerisation of monomers using suitable crosslinking agents and properly functionalising the polymers to suit the synthetic purpose. The proper choice of polymer support is an important step in solid phase organic synthesis. To make solid phase synthesis to be practical, several important issues need to be considered, including the correct choice of solid support, mode of attachment and cleavage of materials from the resin matrix. Efficiency in anchoring and removing a small organic molecule from the polymeric resin relies on the correct choice of the linker group also. This key fragment is crucial in planning a synthetic strategy.
Polystyrene is the most suitable support used for the synthesis of wide range of functionalities including amines. So, for the present investigation the polymer support selected was DVB crosslinked polystyrene of different crosslink density which were prepared by varying the ratio of monomers, DVB and styrene. Using this polymer matrix as the support, the synthesis of spermidine was done . Polystyrene was functionalised to get chloromethylated polystyrene (Merrifield resin). Preparation and attachment of photocleavable linker to the polystyrene matrix, their purification and characterisation, application of the photocleavable linker bearing resin in the synthesis of Spermidine, their purification and characterization were done by C,H,N analysis, 1H NMR, 13C NMR and mass spectrum.
Of the resins, 2% crosslinked polystyrene gave maximum yield and minimum yield was noticed for 4% crosslinked polystyrene resin. This is because 4% crosslinked polystyrene was too rigid to permit easy penetration of reagents causing slower and lesser degree of functionalisation. The 1% crosslinked resin beads were too fragile and disrupted during the photolysis and this made the filtration difficult. As filtration being the main mode of purification, the difficulty in filtration might have led to low percentage yield of synthesised Spermidine while using 1% crosslinked resin.
References
- H.T. Rupniak, D. Paul, J Cell Physiol. 94,161 (1978).
- O.Heby et al. Exp. Cell Res. 90, 8 (1975).
- O.Heby, L.J. Marton, C. B. Wilson, J W.Gray, Eur. J. Cancer 13, 1009 (1977).
- GANEM, B. Acc. Chem. Res. 15, 290 (1982).
- Israel, M. ; Zoll, E.C. ; Muhamed , N.; Modest , E.J. J. Med. Chem. 16,1 (1973).
- Morris, D.R. ; Morton, L. J. Polyamine in Biology and Medicine; Dekkar : Newyork, P. 223 (1981).
- Wang, T; Pankaskie, M.C. ; Fokes, J.E.; Abdel Monem, M.M. Biochem. Biophys. Res.Commn. 94, 85 (1980).
- Feinberg, R. S.; Merrifield, R. B., Tetrahedron Lett. 1974, 3200 (1974).
- Pepper, K. W.; Paisley, H. M.; Young, M. A., J . Chem. Soc. 4097 (1953).
- Baleux, F.; Calas, B.; Mery, J., Int. J. Peptide Protein Res. 28, 22 (1986).
- Sparrow, J. T., J .Org. Chem. 41, 1350 (1976).
- Amundesen, L. H.; Nelson, L. S., J. Am. Chem. Soc. 73, 242 (1951).
- Bruson, H. A., Org. React. 5, 79 (1949).

This work is licensed under a Creative Commons Attribution 4.0 International License.









