Synthesis and Characterization of Palladium(II) Complexes with 2-(2'-hydroxylphenyl)benzoxazole, 2-(2'-hydroxyl phenyl)benzothiazole or the Mixed Ligands
Subhi A. Al-Jibori*, Ibrahim F. Waheed and Ali T. Al-Samaraie
Department of Chemistry, College of Science, University of Tikrit, Iraq.
Corresponding Author: E-mail: subhi_aljibori@yahoo.com
Na2PdCl4 reacts with 2-(2'-hydroxylphenyl)benzoxazole (Hpbo), 2-(2'-hydroxyl phenyl)benzothiazole (Hpbt) or the mixed ligands (Hpbo) and (Hpbt) in methanol to give trans-[PdCl2(Hpbo)2], trans-[PdCl2(Hpbt)2] or trans-[PdCl2(Hpbo)(Hpbt)], respectively the benzoxazole or benzothiazole ligands behave as monodentate ligands coordinated to palladium through the N-heterocyclic atom. Treatment of the prepared Palladium complexes with triethylamine in methanol gave trans-[Pd(pbo)2], trans-[Pd(pbt)2] or trans-[Pd(pbo)(pbt)] respectively. The deprotonated benzoxazole or benzothiazole ligands coordinated to Palladium as bidentate chelate ligands bonded through the N-heterocyclic atom and the anionic oxygen atom of the hydroxyl group. The prepared complexes were characterized by conductivity measurements, the elemental analysis CHN, infrared and 1H n.m.r. spectra.
KEYWORDS:Benzoxazole complexes; Benzothiazole complexes; Mixed ligands; Palladium complexes
Download this article as:| Copy the following to cite this article: Al-Jibori S. A, Waheed I. F, Al-Samaraie A. T. Synthesis and Characterization of Palladium(II) Complexes with 2-(2'-hydroxylphenyl)benzoxazole, 2-(2'-hydroxyl phenyl)benzothiazole or the Mixed Ligands. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Al-Jibori S. A, Waheed I. F, Al-Samaraie A. T. Synthesis and Characterization of Palladium(II) Complexes with 2-(2'-hydroxylphenyl)benzoxazole, 2-(2'-hydroxyl phenyl)benzothiazole or the Mixed Ligands. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23810 |
Introduction
Currently there is a considerable interests in the coordination chemistry of heterocyclic ligands contain nitrogen, oxygen and sulfur donors[1-10]. Partially because of the biological significance of such ligands. Thus benzothiazole derivatives are key components in the bioactive compounds of both natural and synthetic origin. However benzoxazoles are important biological substances, covering an extensive spectrum of essential and selective antimicrobial activities [11-16]. In addition to their biological importance they are strongly coordinating agents and form stable complexes with various transition metal ions [17]. The biological activity of these ligands increase on complex formation with metal ions [18,19]. It is believed that they react selectively toward certain biological system[20].
The use of biological ligands with hard base characters for chelation of heavy metals like uranium, thorium or lanthanides has been a subject of interest of some research groups [21-25] as those kinds of ligands generally produce very stable chelate complexes. This makes them highly appropriates for therapeutic development of clinical chelators in safety procedures related to contamination through natural and depleted thorium and uranium and in similar chemoprevention processes against uranium, thorium heavy metals intoxication.
In view of the above mentioned importance of these ligands and their complexes and as a continuous of our interests in synthesis of new complexes with heterocyclic ligands containing nitrogen, oxygen or sulfur donors [26-30] we report here the syntheses and characterization of palladium(II) complexes with 2-(2¢-hydroxylphenyl)benzoxazole (Hpbo), 2-(2¢-hydroxylphenyl)benzothiazole (Hpbt) and mixed ligands (Hpbo) and (Hpbt).
Experimental
General
The experimental techniques were the same as those used in our recent paper from this laboratory [31].
Starting materials
The compounds 2-(2¢-hydroxylphenyl)benzoxazole, 2-(2¢-hydroxylphenyl) benzothiazole and Na2PdCl4 were commercial products and were used as supplied.
Synthesis of the complexes
trans-[PdCl2(Hpbo)2] (1)
A suspended mixture of Na2PdCl4 (0.074g, 0.25mmol) and Hpbo (0.10g, 0.5mmol) in methanol (5ml) was stirred at room temperature for 3h .The produce yellow solid was filtered off washed with cold methanol and dried under vacuum (0.12g , 82% yield).
Complex 3 was prepared and isolated in a similar manner.
trans-[Pd(pbo)2] (2)
NEt3 (0.02g, 0.2mmol) was added to a suspension of trans-[PdCl2(Hpbo)2] (0.10g, 0.1mmol) in methanol (5ml).The mixture was stirred for 3h during this period the colour of the mixture changed from yellow to orange. The produce orange solid was filtered off washed with cold methanol, dried in a vacuum oven and recrystallized from CHCl3 to give the product as orange crystals (0.08g, 90% yield).
The following complexes were prepared and isolated in a similar manner (4) and (6).
trans-[PdCl2(Hpbo)(Hpbt)] (5)
Methanol (10ml) was added to a solid mixture of Hpbt (0.051g, 0.2mmol), Hpbo (0.046g, 0.2mmol) and Na2PdCl4 (0.059g, 0.2mmol). The resulting mixture was stirred at room temperature for 3h to give a yellow solid, which was filtered off washed with cold methanol and dried in a vacuum oven (0.11g, 89%yield)
Results and Discussion
Synthesis of the complexes
Treatment of Na2PdCl4 with two mole equivalent of 2-(2¢-hydroxylphenyl) benzoxazole (Hpbo) or 2-(2¢-hydroxylphenyl)benzothiazole (Hpbt) in methanol gave complexes of the type trans-[PdCl2(Hpbo)2] (1) or trans-[PdCl2(Hpbt)2] (3) respectively Figure (1). The ligands Hpbo or Hpbt were coordinated as monodentate ligands to palladium through the heterocyclic N-atom. Mixed ligands complex of the type trans-[PdCl2(Hpbo)(Hpbt)] was also prepared by treatment of Na2PdCl4 with one mole equivalent of (Hpbo) followed by (Hpbt).
The prepared complexes are stable at room temperature, soluble in DMSO solvent but are insoluble in other common organic solvent such as CH2Cl2,CHCl3, MeOH or EtOH.
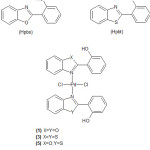 |
Figure 1: structures of the ligands and the prepared complexes |
Complex (1) has been reported previously [32]. It has been made starting with a rather different starting palladium compound, trans-bis(benzonitrile)dichloro palladium(II), and a long reaction time (20 days).
Attempts to displace the chloride ions of the above complexes with saccharinate ion by treatment with sodium saccharinate [33] were unsuccessful and always the starting complexes were obtained. It might be the stirric effect caused by the (hydroxyl groups prevent such reaction to occur.
Treatment of the prepared palladium complexes (1) and (5) with triethylamine in methanol gave the chelated palladium complexes of the type trans-[Pd(pbo)2], trans-[Pd(pbt)2] or trans-[Pd(pbo)(pbt)] respectively Figure(2). The deprotonated anion ligands pbo or pbt behave as chelate ligands coordinated to palladium through the N-atom of the heterocyclic ring at the O-atom of the deprotonated OH group.
The prepared chelated complexes are soluble in CHCl3 or CH2Cl2 but insoluble inMeOH or EtOH solvents.
Attempts to open the chelate ring in the above chelate palladium complexes (2,4,6) with diphosphine such as dppm or dppe in a similar way to that reported for other chelate complexes [26], were unsuccessful and always the starting complexes were obtained.This probably reflect the high stability of chelate ring to be open by diphosphine.
Characterization of complexes
The prepared complexes were identified by Elemental analysis, i.r. spectra, conductivity measurements and 1H nmr. spectra and their data are listed in Tables (1-3). The molar conductivity of complexes in DMSO or CHCl3 is low enough to suggest that they are non-electrolytes [34].
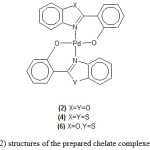 |
Figure 2: structures of the prepared chelate complexes (4)-(6) |
Nuclear magnetic resonance
The 1H-nmr data of the prepared complexes are given in Table 3. The 1H-nmr spectra of complexes trans-[PdCl2(Hpbo)2] (1), trans-[PdCl2(Hpbt)2] (3) showed a broad singlet each at dH=11.20 and 11.54 ppm a singed to the hydroxyl proton in complexes (1) and (2) respectively, while trans-[PdCl2(Hpbo)(Hpbt)] (5) showed two broad singlets at dH=11.18 and 11.58 ppm assigned to two different hydroxyl protons of the two ligands (Hpbo) and (Hpbt) respectively. The phenyl protons appeared within the dH=7.0-8.026 ppm ring. Integration showed that the ratio of the OH: aromatic protons are 1:8.
The hydroxyl signals disappeared in the spectra of the chelated complexes (2), (4) and (6) confirming the deprotontion of the hydroxyl protons. The aromatic protons appeared within the chemical shift dH=6.70-8.0 ppm.
On the basis of the above n.m.r. data and other identification data given in Tables 1 and 2, the structures shown in Figures 1 and 2 have been suggested.
Infrared spectra
The i.r. data of the prepared complexes are given in Table 2. The i.r. spectra of complexes 1, 3 and 5 have a broad medium intensity bond centered at 3299, 3390 and 3284 cm-1 respectively due to the vibration of the hydroxyl group u(OH).
The spectra also showed a strong bond at 338, 342 and 335 cm-1 assigned to u(Pd-Cl) in a trans arrangement around palladium [35,36]. The u(OH) and u(Pd-Cl) bands disappeared in the spectra of complexes (2), (4) and (6) and a new medium intensity band at around 420-433 cm-1 appeared. This band assigned to u(Pd-O) [37]. The spectra of the prepared complexes (1)-(6) showed a medium to strong band at 1596-1606 cm-1 shifted to a lower frequency from that in the corregonding free ligands. This band assigned to u(C=N) of the heterocyclic coordinated to palladium through the N-atom [38]. Further evidence came from the appearance of a new medium intarsity band at 474-485 cm-1 which was absent in the spectra of the ligands this band assigned to u(Pd-N).
Conclusions
In summary reaction of Na2PdCl4 with two mole equivalents of (Hpbo), (Hpbt) or the mixed ligands (Hpbo) and (Hpbt) in methanol gave complexes of the type trans-[PdCl2(Hpbo)2], trans-[PdCl2(Hpbt)2] or trans-[PdCl2(Hpbo)(Hpbt)]. These complexes do not seem to react with sodium saccharinate.
Treatment of the prepared complexes with triethylamine gave the chelate complexes trans-[Pd(pbo)2], trans-[Pd(pbt)2] and trans-[Pd(pbo)(pbt)]. These complexes failed to react with diphosphine such as dppm or dppe.
Table 1: Color, M.P., Yield, Elemental analysis and conductivity of complexes (1)-(6)
|
Λ (ohm-1 . cm2 . mol-1) |
Found(cal.)% |
Yield % |
M.P. Co |
Color |
Compound |
Seq. |
|||
|
CHCl3 |
DMSO |
N |
H |
C |
|||||
|
– |
0.02 |
4.67 (4.7) |
3.03 (3.1) |
52.07 (52.1) |
82 |
288-290 |
Yellow |
[PdCl2(Hpbo)2] |
|
|
0.00 |
– |
5.32 (5.24) |
3.06 (3.07) |
59.27 (59.05) |
91 |
297-300 |
Orange |
[Pd(pbo)2] |
|
|
– |
0.01 |
4.43 (4.14) |
2.87 (2.88) |
49.42 (49.25) |
78 |
256-258 |
Yellow |
[PdCl2(Hpbt)2] |
|
|
0.00 |
– |
5.01 (4.72) |
2.89 (2.70) |
55.87 (55.95) |
94 |
<315 |
Orange |
[Pd(pbt)2] |
|
|
– |
0.01 |
4.55 (4.35) |
2.95 (2.78) |
50.71 (50.78) |
89 |
265-268 |
Yellow |
[PdCl2(Hpbo) (Hpbt)] |
|
|
0.00 |
– |
5.16 (5.31) |
2.97 (2.88) |
57.52 (57.58) |
50 |
285-287 |
Orange |
[Pd(pbo)(pbt)] |
|
Table 2: I.r. spectra date (cm-1) of the ligands and complexes (1)-(6)
|
Compound |
υ(OH) |
υ(Ar-H) |
υ(C=N) |
υ(C=C) |
υ(C-O) |
υas(COC) |
υ(CSC) |
δ(CH) |
υ(Pd-Cl) |
υ(Pd-N) |
υ(Pd-O) |
| Hpbo |
3190b |
3058m |
1631s |
1595s 1485s |
1253s |
1155m |
— |
750s |
— |
— |
— |
| [PdCl2(Hpbo)2] |
3299b |
3064m |
1606s |
1550s 1487s |
1261s |
1197m |
— |
752s |
338s |
485m |
— |
| [Pd(pbo)2] |
— |
3056m |
1604s |
1531m |
1253s |
1193w |
— |
746s |
— |
480m |
420m |
| Hpbt |
3163b |
3000m |
1620s |
1605s 1487s |
1300s |
— |
1106m |
756s |
— |
— |
— |
| [PdCl2(Hpbt)2] |
3390b |
3006m |
1600s |
1508m |
1307m |
— |
1105m |
756s |
342s |
480m |
— |
| [Pd(pbt)2] |
— |
3058m |
1596s |
1542s 1487s |
1290w |
— |
1141w |
748s |
— |
515m |
440m |
| [PdCl2(Hpbo)(Hpbt)] |
3284b |
3070w |
1600m |
1546m 1500m |
1259w 1299w |
1209w |
1108w |
752s |
335s |
474m |
— |
| [Pd(pbo)(pbt)] |
— |
3062m |
1604s |
1537s 1463s |
1251s |
1225w |
1160w |
746s |
— |
480m |
433m |
Table 3: 1H-nmr data for complexes (1)-(6)
|
δH p.p.m. |
Solvent |
Complexes |
Seq. |
|
|
OH |
(phenyl) |
|||
|
11.201 |
8.026-7.078 |
DMSO |
[PdCl2(Hpbo)2] |
1. |
|
— |
7.937-6.721 |
CDCl3 |
[Pd(pbo)2] |
|
|
11.54 |
8.11-7.06 |
DMSO |
[PdCl2(Hpbt)2] |
|
|
— |
7.987-6.960 |
CDCl3 |
[Pd(pbt)2] |
|
|
11.18-11.58 |
8.146-6.973 |
DMSO |
[PdCl2(Hpbo)(Hpbt)] |
|
|
— |
8.001-6.704 |
CDCl3 |
[Pd(pbo)(pbt)] |
|
References
- X-F. He, C.M. Vogels, A. Decken and S.A. Westcott, Can. J. Chem., 2003, 81, 861.
- X-F. He, C.M. Vogels, A. Decken and S.A. Westcott, Polyhedron, 2004, 23, 155.
- R. Bhattacharjce, V. Gayathri and N.M.N. Gowda, Trans. Met. Chem., 2004, 29, 320.
- A. Decken, R.A. Gosage and P.N. Yadav, Can. J. Chem., 2005, 83, 1185.
- B. Machura, R. Kruszynski and J. Kusz, Polyhedron, 2007, 26, 3455.
- C. Xi, Y. Wu and X. Yan, J. Organomet. Chem., 2008, 693, 3842.
- J.A. Moore and J.H. Acquaye, Polyhedron, 2009, 28, 386.
- Y.-P. Tong and Y.-W. Lin, Inorg. Chim. Acta., 2009, 362, 2033.
- D.F. Back, G.M. deOliveira, M.A. Ballin and V.A. Corbellini, Inorg. Chim. Acta., 2010, 263, 807.
- P.S. Verma and and G. Seth, Res. J. of Pharm. Bio. and Chem.Sci., 2012, 3, 435.
- H. Razavi, S.K. Palaninathan, E.T. Powers, R.L Wiseman, H.E. Purkey, N.N. Mohamedmohaideen, S. Deechongkit, K.P. Chiang, M.T.A. Dendle, J.C. Sacchettini, J.W. Kelly, Angew. Chem., 2003, 115, 2864.
- H. Razavi, S.K. Palaninathan, E.T. Powers, R.L. Wiseman, H.E. Purkey, N.N. Mohamedmohaideen, S. Deechongkit, K.P. Chiang, M.T.A. Dendle, J.C. Sacchettini, J.W. Kelly, Angew. Chem., Int. Ed. 2003, 42, 2758.
- J. Koci, V. Klimesova, K. Waisser, J. Kaustova, H.-M. Dahse, U. Mollmann, Bioorg.Med. Chem. Lett. 2002, 12, 3275.
- D.F. Shi, T.D. Bradshaw, S. Wrigley, C.J. McCall, P. Lelieveld, I. Fichtner, M.F. Stevens, J. Med. Chem., 1996, 39, 3375.
- C.J. Paget, K. Kisner, R.L. Stone, D.C. DeLong, J. Med. Chem., 1969, 12, 1016.
- R.T. Daveyjr, R.L. Dewar, G.F. Reed, M.B. Vasudevacharl, M.A. Polls, J.A. Kovacs, J. Falloon, R.E. Walker, H. Masur, S.E. Haneiwich, Proc. Natl. Acad. Sci. USA, 1993, 90, 5608.
- S.E. Castillo-Blum and N. Barba-Behrens, Coord. Chem. Rev., 2000, 3,196.
- N.M. Shivakumaraiah and N. Gowda, Indian J. Chem., 2003, 42A, 1856.
- N. Bharti, M.R. Maurya, L. Naqui and A. Azam, Bio-Org Met. Chem. Lett., 2000, 10, 2243.
- M.A. Ali, A.H. Mirza, M. Nazimuddin, P.K. Dhar and R.J. Butcher, Trans. Met. Chem., 2002, 27, 27.
- E. Bonfada, G. Manzoni de Oliveira, D.F. Back, E. Schulz Lang, Z. Anorg. Allg. Chem., 2005, 631, 878.
- D.F. Back, G. Manzoni de Oliveira, E. Schulz Lang, J. Inorg. Biochem., 2006, 100, 1698.
- D.F. Back, E. Bonfada, G. Manzoni de Oliveira, E. Schulz Lang, J. Inorg. Biochem., 2007, 101, 709.
- D.F. Back, G. Manzoni de Oliveira, J.P. Vargas, E. Schulz Lang, G. Tabarelli, J. Inorg. Biochem., 2008, 102, 666.
- D.F. Back, G. Manzoni de Oliveira, E. Schulz Lang, J.P. Vargas, Polyhedron, 2008, 27, 2551.
- S.A. Al-Jibori, I.N. Al-Nassiri, L.J. Al-Hayaly and T.A.K. Al-Allaff, Trans. Met. Chem., 2002, 27, 191.
- O.H. Amin, L.J. Al-Hayaly, S.A. Al-Jibori and T.A.K. Al-Allaff, Polyhedron, 2004, 23, 2013.
- S.A. Al-Jibori, A.S.S. Al-Zanbi, M.Y. Mahammed and T.A.K. Al-Allaff, Trans. Met. Chem., 2007, 32, 218.
- S.A. Al-Jibori, A.I. Abdullah and T.A.K. Al-Allaff, Trans. Met. Chem., 2007, 19, 1334.
- S.A. Al-Jibori, T.F. Khaleel, S.A. O. Ahmed, L.J. Al-Hayaly, K. Merzweiler, C. Wagner and G. Hogath, Polyhedron, submitted.
- A.S.M. Al-Janabi, B.H. Abdullah and S.A. Al-Jibori, Orient. J. Chem., 2009, 25, 277.
- M. Ito, A. Furuhashi and M.Shimoi ,Polyhedron, 1997, 16, 1889.
- W. Henderson, B.K. Nieholson and L.J. Mccaffery, Inorg. Chim. Acta., 1999, 285, 145.
- W.J. Geary. Coord. Chem. Rev., 1971, 7, 81.
- D. Brown; R. Burbank, M. Robin., J. Am. Chem. Soc., 1969, 91, 2895.
- K. Nakamoto, “In Infrared and Raman Spectra of Inorganic and Coord ination Compounds” 5th ed. Wiley: New York, (1997).
- M. Biyala, N. Fahmi, R. Singh, Indian J. Chem., 2004, 43A, 2536.
- M. Samota, G. Seth, J. Hetero. Chem., 2010, 21, 44.

This work is licensed under a Creative Commons Attribution 4.0 International License.









