Synthesis and Characterization of Nano-Sized CeO2 in the Presence of Nonionic Surfactant and by Different Precipitation Agents
Masoumeh Tabatabee and Reyhaneh Sheykhalishahi
Department of Chemistry, Yazd Branch, Islamic Azad University, Yazd, Iran.
Corresponding Author E-mail: tabatabaee45m@yahoo.com
Nano-sized cerium oxide (CeO2) was synthesized successfully by a simple method using polyethylene glycol (PEG 2000) as a nonionic surfactant and different precipitating agents.X-ray diffraction (XRD) scanning electron microscope (SEM) and transmission electron microscope (TEM were used to characterize the structure and morphology synthesized powder. The nanocrystaline CeO2 was grown in face-centered cubic. Sample prepared by triethylamine as precipitating agent possess spherical nano-sized particle with the average crystallite size 25 nm.
KEYWORDS:CeO2; Nanoparticles; Polyethylene glycol; Precipitation
Download this article as:| Copy the following to cite this article: Tabatabee M, Sheykhalishahi R. Synthesis and Characterization of Nano-Sized CeO2 in the Presence of Nonionic Surfactant and by Different Precipitation Agents. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Tabatabee M, Sheykhalishahi R. Synthesis and Characterization of Nano-Sized CeO2 in the Presence of Nonionic Surfactant and by Different Precipitation Agents. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23801 |
Introduction
In the past few years, much attention has been focused on the research field of nano-crystalline metal oxides both because of their fundamental importance and the wide range of potential technological applications (1-6). Rare earth oxides are used as a component in various optical, electrical and magnetic applications such as optical wave guide optical filters and capacitors (7). These applications are based on particle size and morphology of metal oxides. Nano particles of lanthanide groups are used in many fields, including optics,
biological labeling, magnetism, electrode materials and conductive ceramics (8). Cerium oxide (ceria) is a semiconductor with high photocatalytic capability and ceria nanoparticles have attracted attention within the biomedical research community as a potential agent to inhibit cellular aging (9). A number of studies have reported that the presence of PEG can modify or control the surface of the nanometer crystal, moreover can act as the dispersing agent of the nanometer crystal in the process of synthesis (10, 11). In the present investigation, we compare the synthesis of nano crystalline CeO2 by different precipitants such sodium hydroxide, ammonia and triethylamine from Cerium nitrate in the presence of Polyethylene glycols.
Experimental procedure
Fig 1 shows a schematic diagram of the synthesis procedure. Cerium nitrate hexahydrate, Ce(NO3)2·6H2O (1 g) and polyethylene Glycol (3 g, average molecular weight 2000, abbreviated as PEG2000) dissolved in distilled water (20 mL). The pH value of the mixture was adjusted to 12 by sodium hydroxide, ammonia and triethylamine (TEA) (the solutions were labeled as A, B and C respectively). The reaction mixtures refluxed at 150 °C for 2 h. The resulting precipitate was centrifuged and washed with distilled water and ethanol to remove polyethylene Glycol and other impurities. The resulted powders were dried in 100 °C and calcinated at 600 °C for 2 h.
The prepared powders were characterized by powder X- ray diffraction (Bruker, Advance D8) with Cu Kα (λ=1.5406 Å) incident radiation. The size distribution and morphology of the samples were analyzed by scanning electron microscopy (SEM, Philips XL30) and Transmission electron microscopy (TEM, Philips CM10).
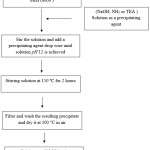 |
Figure 1: Schematic diagram of the synthesis procedure for CeO2 nanoparticles. Click here to View figure |
Results and discussion
Figure 2 illustrates the XRD patterns of the prepared powders. The crystal structures of all synthesized is Face-centered cubic and the entire d-line patterns match with reported values (JCPDS Card Pattern: 04-0593 for A, 43-1002 for B and C). The particle size is calculated Debye-Scherer formula, D=kλ/βcosθ where D is the crystallite size, k is a constant (=0.9 assuming that the particles are spherical), λ is the wavelength of the X-ray radiation, β is the line width (obtained after correction for the instrumental broadening) and θ is the angle of diffraction. The average particle size obtained from XRD data is ~30 nm for A, ~50 For B and For ~20 C.
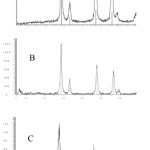 |
Figure 2: The XRD patterns of CeO2 nanoparticles obtained from (a) NaOH, (b) NH3 and (c) TEA, cerium nitrate solutions and in the presence of POG. Click here to View figure |
The SEM and TEM images of sample prepared by triethylamine as precipitating agent is shown in Figs. 3 and 4 respectively. The TEM micrograph shows clearly that the particle size of nano-sized CeO2 is ~25 nm. This result is in well agreement with the crystallite size calculated from Debye-Scherer formula.
Calculation
Nanocrystalline CeO2 powders are obtained in the presence of poly ethylene glycol as a neutral surfactant and different precipitating agents. Results show sample prepared by triethylamine as precipitating agent possess spherical nano-sized particle with smallest crystalline size.
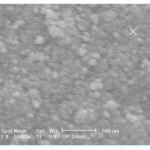 |
Figure 3: SEM micrograph of CeO2 powders prepared in the presence of triethylamine as precipitating agent. Click here to View figure |
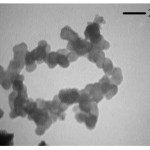 |
Figure 4: TEM micrograph of CeO2 powders prepared Fig. 2 SEM micrograph of CeO2 powders prepared in the presence of triethylamine as precipitating agent. Click here to View figure |
References
- Cushing B. L. Kolesnichenko V.L. and ÓConnor C. J. Chem. Rev., 104, 3893 (2004).
- Waghulade R. B. Patil P.P. and Pasricha R. Talanta, 72, 594 (2007).
- Chaudhari G.N. Bende A. M. Bodade A. B. Patil S. S. and Sapkal V.S. Sens. Actuators B, 115, 297 (2006).
- Srivastava A. Jain K. Rashmi, Srivastava A. K. and Lakshmikumar S. T. Mater. Chem. Phys., 97, 85 (2006).
- Chaudhari G. N. Bende A. M. Bodade A. B. Patil S. S. and Manorama S.V. Talanta, 69, 187 (2006).
- Chen A. Huang X. Tong Z. Bai S. Luo R. and Liu C. C. Sens. Actuators B, 115, 316 (2006).
- Vadivel M. A. Navale S. C. and Ravi V. Mater. Lett., 60, 848 (2006).
- Li G. Peng C. Zhang C. Xu Z. Shang M. Yang D. Kang X. Wang W. Li C. Cheng Z. Lin J., Inorg. Chem., 49, 2010, 10522.
- Rzigalinski B., Tech. Canc. Resea. & Treatm., 4, 651 (2005).
- Qiu C. Xiao X. and Liu R., 34, 1747(2008).
- Duan J. X. Huang X.T. and Wang E., Mater. Lett., 60, 1918 (2006).

This work is licensed under a Creative Commons Attribution 4.0 International License.









