Spectrophotometric Determination of Colchicine by Phase Transfer Catalyst
Ali Daneshfar, Farbod Ansari and Siavash Hasanvandi*
North Drilling Company (NDC, Tehran) Drilling Fluids Department, Postal Code: 1467894113, Tehran, Iran.
Corresponding author: E-mail: Siavash_hasanvandii@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/280113
a simple, rapid, and with high specificity method has been proposed for the determination of colchicine from biological samples as well from tablet. The method based on the reaction of the colchicine with quaternary ammonium salt in aqueous solution. The analytical parameters such as type of organic solvent (extraction solvent), volume of aqueous solvent, type of salt (ion-pairing agent), concentration of salt and effect pH have been investigated. The calibration graph was linear in the range of 0.3-6 µg mL-1 with the correlation coefficient of 0.999 under the optimum conditions. Good repeatability (R.S.D, %) 2.45, 1.33 and 2.36 for urine and 2.20, 1.97 and 2.79 for plasma in three spiked level 5, 2 and 0.5 µg mL-1 respectively and 2.26 for tablet was obtained.
KEYWORDS:Colchicine; ion pair; extraction; phase transfer catalyst
Download this article as:| Copy the following to cite this article: Daneshfar A, Ansari F, Hasanvandi S. Spectrophotometric Determination of Colchicine by Phase Transfer Catalyst. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Daneshfar A, Ansari F, Hasanvandi S. Spectrophotometric Determination of Colchicine by Phase Transfer Catalyst. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23756 |
Introduction
Colchicine is an alkaloid that has been used for centuries in acute gouty arthritis. Recently it has been employed for an increasing number of diseases and disorders [1-6]. The drug also presents an antiniotic action; arresting dividing cells in metasolvent by preventing normal function of miotic spindle, which helps cell divide. Colchicine is an alkaloid with the chemical formula N-(5, 67, 9, tetrahydro-1, 2, 3, 10, tetrametoxy-9 oxobenzo[a] hep-tain-7-yl) acetamide whose structure is shown in Fig. 1. Different methods have been employed to determination of colchicine including, spectrophotometric [7], volumetric [8], potentiometric [9], voltametric [10], radio and enzyme immunoassays [11], and other methods, each of them presenting a series of advantages and disadvantages. The present study describes accurate extraction spectrophotometric method for the determination of colchicine through ion-pair complex formation with quaternary ammonium salt (Phase transfer catalyst). Phase transfer catalyst (PTC) was first introduced in the 1960s, as a tool to efficiently perform reactions between water soluble inorganic reagents and organic substrates, dissolved in mutually Immiscible liquid solvents. Moreover, the term PTC encompasses several different techniques characterized by operational, Simplicity, mild conditions, high reaction rates, high selectivity, and the utilization of inexpensive reagents [12-14]. Already, Ion-pair formation between a negative charged aromatic ion and positive phase transfer catalyst is reported [15,16]. NMR spectrum of colchicine revealed that bond length between oxygen and carbon on aromatic cycle (tropylium oxide) is longer than double bond and dipole ion in which negative charge placed on oxygen atom is formed [17], As shown in Fig. 2. Other evident proves that dipole structure of the molecule, solubility in water (10 µg mL−1) with molar mass, 399.44. The other structure, colchinol N-acetyl methylether (Fig. 3) with similar functional groups slightly soluble in water. This is revealed that formed dipole responsible of solubility of molecule. Therefore, ion-pair can be formed between this negative ion and a quaternary ammonium salt.
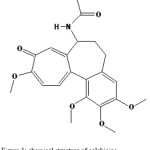 |
Figure 1: chemical structure of colchicine |
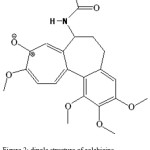 |
Figure 2: dipole structure of colchicine. |
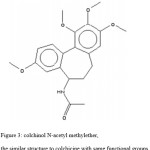 |
Figure 3: colchinol N-acetyl methylether, the similar structure to colchicine with same functional groups. |
Exprimental
Reagent and solutions
All of the chemical used were of analytical grade and used without further purification. Double distilled ionized water was used to prepare all solution. Colchicine was obtained from Sigma, Aldrich. 2-ethyl-1-hexanol, decanol, isobuthyl methyl ketone, ethyl acetate, tetrapropylammonium bromide (TPAB), tetraethylammonium chloride (TEAC) and cetyl terimethylammonium bromide (CTAB) were purchased from Merck (Darmstadt, Germany). Dichloromethane and chloroform were obtained from Acros.
Apparatus
All absorbance spectral measurements were made using double beam cary 300 BIO UV-VIS spectrophotometer (Varian Australian pty LTD) with wavelength range 190-800 nm, spectral bandwidth 1.5 nm , with 10 mm matched quartz cell. IKARCT basic magnetic stirrer, RCTB model with stirring rate up to 1500 rpm. Startious balance with Readability, 0.0001g.
Samples
Stock solutions of colchicine of double distilled ionized water freshly prepared each day and were stored in the dark, because of the colchicine is strongly degraded under light exposure, the absorbance spectra completely different after 2 day of light exposure of the colchicines̀ s solutions [18].
Procedure
Extraction experiment was performed using 20 ml of 2 µg mL−1 colchicine in aqueous solution with pH 4 (using H3PO4-KOH buffer) and 0.1 mmol mL-1 of TPAB in 50 ml flask. Then 4 ml of 2-ethyl-1-hexanol as extraction solvent is added and the content were shaken for few seconds. Separation of solvents was carried out by centrifugation for 3 min at 2500 rpm. 3.5 ml of organic solvent after centrifugation was withdrawn into auto sampler and then transferred to 1cm quartz cell. Then colchicine concentration was determined spectrophotometrically. The drug was extracted and measured quantitatively with maximum absorbance at 353 nm.
Results and discussions
In order to obtain the most effective extraction, it is important to determine the optimum conditions. These conditions include the type of organic solvent (extraction solvent), the volume of aqueous solvent, the type of the ion-pairing agent, the concentration of the salt (TPAB) and effect of pH.
Selection of extraction solvent
The choice of an appropriate extraction solvent is essential in liquid-liquid extraction in order to obtain efficient extraction. Different organic solvent as dichloromethane, chloroform, 2-ethyl-1-hexanol, decanol, isobuthyl methyl ketone and ethyl acetate were tested as extractive solvent. The organic solvent must be immiscible with water, nonvolatile to prevent of decrease of organic solvent during extraction procedure and have extraction capability of the interested compound. Also are less harmful to environment. Dichloromethane and chloroform were preferred to other solvents because of obtained highest absorbance but dichloromethane is a volatile and both dichloromethane and chloroform are toxic solvents. As can be seen in Fig. 4, Difference between observed absorbance of recent solvents and 2-ethyl-1-hexanol is much less and ignorable. 2-ethyl-1-hexanol is a nonvolatile solvent and less harmful to environment than Dichloromethane and chloroform. Study of chloroform and dichloromethane due to investigation of extraction capability of interest analyte by these two solvents compare with other extraction solvents. Thus, 2-ethyl-1-hexanol chooses as extraction solvent.
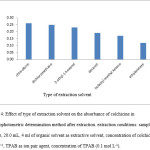 |
Figure 4: Effect of type of extraction solvent on the absorbance of colchicine in Spectrophotometric determination method after extraction. extraction conditions: sample volume, 20.0 mL, 4 ml of organic solvent as extractive solvent, concentration of colchicine, 2.0 µg mL−1, TPAB as ion pair agent, concentration of TPAB (0.1 mol L-1). |
Effect of aqueous solvent volume
A series of volumes (4-20 ml) of aqueous solvent were used to estimate the influence of different volumes of aqueous solvent on the extraction. It is clear that the aqueous solvent containing colchicine increases while organic solvent volume is remained constant result in increasing of concentration of colchicine in extraction solvent and subsequently increase it absorbance. As shown in Fig. 5, when aqueous solvent volume was greater than 20 ml a deviation of linearity in graph of absorbance versus aqueous solvent volume is occur. Accordingly, 20 ml of aqueous solvent was selected as aqueous solvent volume. 24 ml of a binary mixture of aqueous solvent and extraction solvent in ratio of (5:1) has been developed for extraction of colchicine.
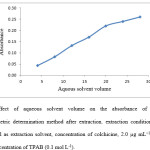 |
Figure 5: Effect of aqueous solvent volume on the absorbance of colchicine in Spectrophotometric determination method after extraction. extraction conditions: 4 ml of 2-ethyl-1-hexanol as extraction solvent, concentration of colchicine, 2.0 µg mL−1, TPAB as ion pair agent, concentration of TPAB (0.1 mol L-1). |
Effect of salt type (quaternary ammonium salt)
The effect of salt type (quaternary ammonium salt) on the extraction of colchicine from aqueous solvent was studied. It was noticed that the maximum absorbance intensity were observed in presence of TPAB as quaternary ammonium salt. The result shows in Fig. 6.
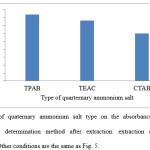 |
Figure 6: Effect of quaternary ammonium salt type on the absorbance of colchicine in Spectrophotometric determination method after extraction. extraction conditions: sample volume, 20.0 mL. Other conditions are the same as Fig. 5. |
Effect of TPAB concentration
The effect of salt concentration on intensity of the absorbance at selected wavelength was tested using various concentration of TPAB (1-3-1 mmol mL-1) and its effects on the extraction process are shown in Fig. 7. As can be seen, the efficiency of colchicines transport increases with increasing TPAB concentration until 0.1 mmol mL-1 is reached. However, a further increase in the concentration of TPAB (up to 0.2 mmol mL-1) caused a decrease in the absorbance. Hence, 0.1 mmol mL-1employed as the optimum concentration of TPAB.
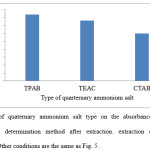 |
Figure 7: Effect of concentration of TPAB on the absorbance of colchicine in Spectrophotometric determination method after extraction. Extraction conditions are the same as Fig. 5.
|
Effect of pH
The effect of pH on the extraction of colchicine was studied within the range of 1-13. The effect of pH was studied by extracting the ion-pair complex in presence of H3PO4-KOH buffer. Dependence of extraction efficiency probably due to followed reasons. When H3PO4 concentration increases, the negatively charged oxygen atom in aromatic structure of colchicine is protonated and ion-pair complex is not formed. By increasing of pH, TPAB attracted with hydroxide ions and ion-pair complex is not allowed to form. The results illustrated in Fig. 8 show that the absorbance is nearly constant in the range of 4-7. In order to obtain high extraction efficiency pH 4 was chosen.
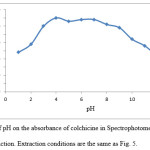 |
Figure 8: Effect of pH on the absorbance of colchicine in Spectrophotometric determination method after extraction. Extraction conditions are the same as Fig. 5. |
Effect of interferences
The effect of various ions on the determination of 2 µg mL−1 colchicine was investigated. A given species was considered to interfere if result in ±5 percentage variation of the absorbance signal. The results obtained is presented in Table 1. As can be seen, the effect of various ions is negligible and colchicine can be determined quantitatively in real samples without interferences from matrix of the samples.
Table 1: effect of foreign ions on determination of colchicine
|
2000 |
1000 |
Concentration (mg L-1) |
|
NO-3, Na+ |
HCO-3, CO3-2, K+, I–, Fe+2 Mn+2, Zn+2, ClO3–, Br–, SO4-2 NH4+, SCN–, HPO4-2, Ca+2 F–, Cu+, Cl–, PO4-3 |
foreign ions |
Method validation
Analytical figure of merits
To evaluate the practical applicability of the method for determination of colchicine, the figures of merit including linear dynamic range (LDR), limit of detection(LOD), limit of quantification (LOQ) and extraction recovery for the extraction of the drug from the aqueous solutions were investigated under the must appropriate conditions.
Linearity and range
Calibration curve was plotted using nine spiked level of the drug in the concentration ranging from 0.3-6 µg mL−1 with the correlation coefficient of 0.999. For each level, at least three replicate extractions were performed. The correlation coefficient was indicating good linearity.
Detection limit and quantification limits
Detection limit (LOD) for the method was calculated using the following equation:
![]()
Where Sb is the standard deviation of ten replicate determination values under the same conditions as for the sample analysis in the absence of the drug and k is the sensivity, namely the slop of the calibration graph. In accordance with the formula, the detection limit was found to be 0.07 µg mL−1. The limit of quantification, LOQ defined as
![]()
According to this equation, the limit of quantification was found to be 0.23 µg mL−1. The obtained Results are presented in Table 2.
Table 2: Analytical characteristics of determination of colchicine
| Parameters | Analytical feature |
| Linear range (µg mL−1) | 0.3-6 |
| r2 | 0.999 |
| Limit of detection (µg mL−1) | 0.07 |
| SD of 10 blank measurements (µg mL−1) | 5×10-3 |
| Limit of quantification (µg mL−1) | 0.23 |
| Repeatability (R.S.D, %) | 1.46 |
Recovery
Plasma and urine analysis
Extraction of colchicine from human plasma and urine due to the importance of the analysis of drugs in biological samples was carried out. The method was applied to determine concentration of colchicine in drug free plasma and urine samples. For preparation of biological samples, the follow procedure was applied. 20 ml aliquot of the plasma sample was added to a flask followed by the addition of 40 ml of acetonitrile and shaken for few second. Then 30 ml of this mixture was transferred into screw cap falcon test tube and then centrifuged at 5000 rpm for 10 min. 20 ml of this solution used for next step. 20 ml aliquot of the urine sample was added to a flask followed by dilution with 40 ml of deionized water- acetonitrile (50:50 v/v) and shaken for few seconds. Then 30 ml of this mixture was transferred into screw cap falcon test tube and then centrifuged at 5000 rpm for 10 min. 20 ml of centrifuged solution used for urine analysis. Same extraction procedure was applied to determination of drug in real samples. Plasma and urine samples were spiked with colchicine at three spiked level 0.5, 0.2 and 5 µg mL−1 as can be seen in table 3. The relative recoveries of plasma at spiking level 0.5, 0.2 and 3 µg mL−1 were 97, 97 and 95 % and for urine at three spiked level 0.5, 0.2 and 5 µg mL−1 were 96, 97 and 94 respectively. The method was successfully applied to determination of colchicine in their commercially tablets. The applicability of method for the assay of the cited drug in pharmaceutical formulation was examined and the result was tabulated in Table 3. The result was reproducible with low R.S.D. % values. The average percent recoveries obtained for tablet were quantitative (0.97), indicating good accuracy of method. The result of analysis of commercial tablets and recovery study of biological samples suggested that there is very less interference from foreign ions, which are present in real samples.
Table 3: Evaluation of accuracy and precision of the proposed method
|
R.S.D, % |
Average recovery, % |
Recovery, % |
Added colchicine (mg L-1) |
samples |
|
2.20 |
0.96 |
0.95 |
5 |
plasma |
|
1.97 |
|
0.97 |
2 |
|
|
2.79 |
|
0.97 |
0.5 |
|
|
2.45 |
0.96 |
0.94 |
5 |
urine |
|
1.33 |
|
0.97 |
2 |
|
|
2.36 |
|
0.96 |
0.5 |
|
|
2.26 |
0.97 |
0.97 |
— |
tablet |
Conclusion
A Spectrophotometric determination method by solvent transfer catalysts was developed for determination of colchicines in both biological and tablets samples. Parameters such as type of organic solvent, aqueous solvent volume, type of salt, concentration of salt and pH were investigated. Due to use of 2-ethyl-1-hexanol as extraction solvent, the method less harmful to environment. The proposed method seems to be more preffered for its simplicity, analytical precision and improved extraction selectivity. Ion pair extraction, has received widespread attention for it high selectivity, utilization of inexpensive reagents and so on. it still has attracted considerable scientific and practical interest. Therefore, it can be useful for routine analyses and quality control assay of the examined drug in biological material and in tablets without fear of interference caused by excipients expected to be presented in real samples.
References
- Kaplan, M. N., Alling, D.W., Zimmerman, H. S., Wolfe, H. J., Sepersky, R. A. and Hirsch, G. S., N. Engl. J. Med., 315, 1448 (1986).
- Seidman, E., Fjellner, B. and Johannesson, A., J. Rheumatol., 14, 777 (1987).
- Takigawa, M., Myachi, M. and Tagami, H., Arch. Dermatol., 118 458 (1982).
- Hazen, P. G. and Michel, B., Arch. Dermatol., 115, 1303 (1979).
- Tones, M. D. and Furst, D. E., Rheum. Dis. Clin. North Am., 16, 217 (1990).
- Kaplan, H., N. Engl. J. Med., 213, 774 (1960).
- Schmit, J., M. Bull. Trav. Soc. Pharm. Lyon., 12, 31 (1968).
- Karawya, M. S. and Diab, A. M., J. AOAC., 58, 1171 (1975).
- Bodoki, E., Săndulescu, R. and Roman L., C. E. J. C., 5, 766 (1998).
- Bersier, P.M., J. Bersier, Electroanalysis, 6, 171 (1994).
- Poulev, A. Deus-Neumann, B., Bombardelli, E. and Zenk, M. H., Planta. Med., 60, 77 (1994).
- kosza, M. M. and Serafin, B., Rocz. Chem., 39, 1223 (1965).
- Bra¨ndstrom, A. and Gustavii, K., Acta Chem. Scand., 23, 1215 (1969).
- Starks, C. M., J. Am. Chem. Soc., 93, 195 (1971).
- Yiannis, C. F., Kefala, A. P. and Stalikas, C. D., J. Chromatogr. A., 1190, 44 (2008).
- Yang, H. M. and Huang, C. C., Applied Catal. A., General 299, 258 (2006).
- Macia˛z˙ek-Jurczyk, M., Sułkowska, A., Bojko, B., Rownicka-Zubik, J. and Sułkowski, W. W. Spectrochim. Acta Part A., 74, 1 (2009).
- Karawya, M. S. and Diab, A. M., J. AOAC., 58, 1171 (1975).

This work is licensed under a Creative Commons Attribution 4.0 International License.









