Enviromentaly Friendly Functionalization of Carboxylated Shortend Multi-Wall Nanotubes with Sunset Yellow Dye
Javad Azizian*, Shahab Zomorodbakhsh, Abolghasem Shameli and Mahdieh Entezari
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding author: E-mail: j-azizian@cc.sbu.ac.ir.
In this paper, the chemical functionalization of carboxylated shortend multi-walled carbon nanotubes (MWNT-COOH) by sunset yellow dye via esterification method in water have been investigated.The functionalized MWNTs were characterized by Fourier Transform Infrared spectroscopy (FT-IR), Raman spectroscopy, elemental analysis,scanning electron microscopy (SEM), Defunctionalization test and UV analysis.
KEYWORDS:Functionalization; Shortend Multi wall nanotubes; Sunset yellow
Download this article as:| Copy the following to cite this article: Azizian J, Zomorodbakhsh S, Shameli A, Entezari M. Enviromentaly Friendly Functionalization of Carboxylated Shortend Multi-Wall Nanotubes with Sunset Yellow Dye. Orient J Chem 2012;28(1). |
| Copy the following to cite this URL: Azizian J, Zomorodbakhsh S, Shameli A, Entezari M. Enviromentaly Friendly Functionalization of Carboxylated Shortend Multi-Wall Nanotubes with Sunset Yellow Dye. Orient J Chem 2012;28(1). Available from: http://www.orientjchem.org/?p=23764 |
Introduction
Sunset yellow is an azo compound that is found in shoe polish, industrial products and common food products such as candies, beverages and bakery products. This dye has a nitrogen to nitrogen double bond as its chromophore 1-2. The group attach to the surface of carbon nanotube, in order to take advantage of its outstanding mechanical, optical, solubility, electrical and thermal properties. Carbon nanotubes have unique properties that make them attractive for different engineering applications and many other fields 3-7. multiwalled carbon nanotubes are more attractive than singlewalled carbon nanotubes because of their relatively low production costs and availability in large quantities.
However, because of their chemical inertness, carbon nanotubes have to be functionalized in order to acquire additional physico-chemical properties8. These groups, which are chemically attached to the tubes, are mostly represented by –COOH groups, less by –C=O, and –OH groups 9-10. In this paper we present a simple route to attach Sunset yellow to MWNT–COOH via esterification method (Scheme 1). The product was characterized by spectroscopy (FT-IR), Raman spectroscopy, elemental analysis,scanning electron microscopy (SEM), Defunctionalization test and UV analysis.
Experimental
All reagents and solvents were obtained from Merck Chemical Inc. (Darmstadt, Germany), and MWCNT–COOH (95% purity, 20–30 nm; Netvino Co. Ltd) were purchased and used as received. The FT-IR spectra were recorded using KBr tablets on a Nexus 870 FT-IR spectrometer (Thermo Nicolet, Madison, WI). FT-Raman spectra were recorded on 960 ES, 1064 Thermo Nicolet. SEM measurement was carried out on the XL30 electron microscope (Philips, Amsterdam, Netherlands) for study the morphology of the MWCNTs. Elemental analyses of carbon, hydrogen, nitrogen and sulphur were performed using a Series ΙΙ 2400(Perkin Elmer, Waltham, MA) and UV-visible spectra were recorded on a UV-Visible spectrometer (GBC Cintra 20, Victoria, Australia).
Preparation of MWNT- Sunset yellow
60 mg MWNT–COOH (20–30 nm; Netvino Co. Ltd) were sonicated in 50 mL of deionized water for 30 minutes to give a homogeneous suspension, sunset yellow dye
(180 mg) and a few drops of sulfuric acid as a catalyst were added to the MWNT–COOH suspension at room temperature. The mixture was stirred for two hours. Finally, the temperature was increased to 90°C and the mixture was stirred for 2 day. after completion of the reaction, the mixture was poured into 250 ml of Ethanol and vacuum-filtered through a filter paper (3 μm porosity). This washing operation was repeated five times and followed by washing with petroleum ether three times. The product was then washed with deionized water and acetone, and the functionalized MWCNT was dried in a vacuum oven at 90°C. Figure 1 shows the synthesis route of the modified MWNT- Sunset yellow
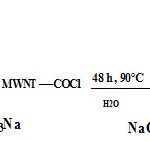 |
Figure 1 |
Results and Discussion
Fig.2 shows the FT-IR spectrum of the modified MWNTs. In spectrum A, the band at around 1527 cm-1 corresponds to the stretching mode of the C =C double bond that forms the framework of the carbon nano tube Sidewall 11. The peaks at 1704, 3304 cm-1 and 1071 cm-1 apparently corresponds to the stretching modes of the carboxylic acid groups 12. In spectrum B The carbonyl peak shift to 1739 cm-1 (as compared with 1704 cm-1 in spectrum A) is a result of esther (C =O)OR linkage formation and the peaks at 3304 (OH) is removed.
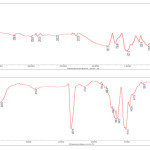 |
Figure 2: Fourier transform spectra of A; carboxylated shortend multi-walled carbon nanotube (MWNT-COOH) and B; modified multi-walled carbon nanotube ( MWNT- Sunset yellow) |
Raman spectroscopy is a powerful tool used to provide structural information about MWNT–COOH before and after functionalization. As shown in Fig.3, the D and G bands of the MWNT at around 1339 and 1596 cm, attributed to defects, disorder-induced peaks, and tangential- mode peaks,(A-B) can be clearly observed for MWNT–COOH and MWNT– Sunset yellow. Additionally, the intensity ratio (ID/IG) of the D and G bands for MWNT– Sunset yellow (B) is 1.51 which is greater than that for MWNT–COOH (A) 0.54. The increase in intensity of the defect mode at 1339 cm was related to sp3 hybridization of carbon, and is used as an evidence of the disruption of the aromatic system of π electrons by the attached molecules. 13-16
 |
Figure 3: Raman spectroscopy of A; carboxylated shortend multi-walled carbon nanotube (MWNT-COOH) and B; modified multi-walled carbon nanotube ( MWNT- Sunset yellow) |
Elemental analyses of the MWNT-COOH (A) and MWNT- Sunset yellow (B) are shown in Table 1. Apart from the carbon values, the atomic percentages of hydrogen, nitrogen and sulphur of B (as compared with A) indicated that A is functionalized with sunset yellow dye.
Table 1: Elemental analysis of A; carboxylated shortend multi-walled carbon nanotube (MWNT-COOH) and B; modified multiwalled carbon nanotube ( MWNT- Sunset yellow)
| Sample | %C | %H | %N | %S |
| A | 96.4 | 0.04 | 0 | 0 |
| B | 87.8 | 0.2 | 1.4 | 1.1 |
The morphology of the resulting MWNT- Sunset yellow was observed with SEM. In Fig 4, SEM images of Aand B are shown. It indicates that the MWNT–COOH (A) has a smooth surface. The changes in the morphology for MWNT- Sunset yellow (B) is remarkable. A uniform tubular layer due to esther group on the surface of the MWNT (the rough part) is observable. It seems that the diameters of B is slightly increased in comparison to A 17-21.
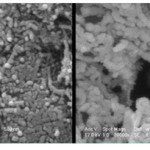 |
Figure 4: Scanning electron microscopy images of A carboxylated multiwalled carbon nanotubes and B MWCNT-Sunset yellow. |
Defunctionalization
The carbon nanotubes can be recovered from the soluble samples via defunctionalization. The characterization of the defunctionalized samples provides
further evidence for the conclusion that the soluble samples before defunctionalization contain substantial amount of carbon nanotubes. In addition, the gravimetric results associated with the defunctionalization allow an estimate of the carbon nanotube contents in the soluble samples. For soluble carbon nanotube samples that are based on the formation of ester linkages, a classical example for chemical defunctionalization is acid- or base-catalyzed hydrolysis, which removes the functional groups from the nanotube surface and results in the precipitation of defunctionalized carbon nanotubes. For example, for the solubilized carbon nanotube MWNT- Sunset yellow, the acid-catalyzed hydrolysis can be accomplished by refluxing the soluble samples with a strong acid such as trifluoroacetic acid.22 The hydrolysis reaction in homogeneous solution yields defunctionalized carbon nanotubes as dark-colored precipitates. The high content of carbon nanotubes in the defunctionalized samples makes it possible to carry out elemental analyses (Fig 5).
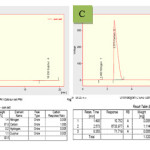 |
Figure 5: Elemental analysis of B; Functionalized multiwalled carbon nanotube ( MWNT- Sunset yellow) and C; Defunctionalized MWNT- Sunset yellow. |
As a result of this functionalization, the solubility of MWNT- Sunset yellow was improved significantly, and they were easily dispersed in deionized water.A dispersion test gives a fair idea whether the modification on the carbon nanotubes has been achieved or not. Figure 6 presents a photograph of two vials containing MWNTCOOH
and MWNT- Sunset yellow dispersed in deionized water. As can be seen from Figure 6,
MWNT-COOH are insoluble in deionized water while the modified CNTs can be directly dispersedin deionized water (without sonication) homogeneously and no precipitation was found even after it was sealed for 2 month at room temperature.
 |
Figure 6: The images of MWNT-COOH and MWNT- Sunset yellow in deionized water (1mg/6ml) after standing for 2 month. |
Conclusions
functionalized carbon nanotubes have played and will continue to play an important role in the research and development of nanotubes-based materials and systems. Carbon nanotubes can be functionalized via esterification of the nanotube-bound carboxylic acids. A detailed methodology for the modification and functionalization of multiwalled carbon nanotube (MWCNT) via Esterification has been presented. The solubility of the carbon nanotubes associated with the functionalization and chemical modification offers excellent opportunities not only in the characterization and understanding of carbon nanotubes but also in the utilization of carbon nanotubes for various nanomaterials.
Acknowledgements
The authors are grateful to the Department of Chemistry, Sience and Research Branch, Islamic Azad University,Tehran, Iran for the financial support.
References
- Sayar S, Özdemir, Y. Food Chemistry, Volume 61, Issue 3, 31 March 1998, Pages 367-372
- Kimura M, Umemoto M, Tsuji S, Shibata T, Ito Y. Eisei Shikenjo Hokoku Bulletin Of National Institute Of Hygienic Sciences (1993) Issue: 111, Pages: 137-138.
- M. Dresselhaus, G. Dresselhaus, and P. Avouris, Carbon Nanotubes: Synthesis, Structure, Properties, and Applications. Germany: Springer-VerlagBerlin HeidleberG, 2001.
- S. J. Pastine, D. Okawa, B. Kessler, M. Rolandi, M. Llorente, A. Zettl, and J. M. J. Frechet, “A Facile and Patternable Method for the Surface Modification of Carbon Nanotube Forests Using Perfluoroarylazides”, JACS, 2008), 130, 4238–4239.
- R. Hu, B. A. Cola, N. Haram, J. N. Barisci, S. Lee, S. Stoughton, G. Wallace, C. Too, M. Thomas, A. Gestos, M. E.dela Cruz, J. P. Ferraris, A. A. Zakhidov, and R. H. Baughman, “Harvesting Waste Thermal Energy Using a Carbon-Nanotube-Based Thermo-Electrochemical Cell”, Nano Lett., 2010, 10 , 838–846.
- M. S. Arnold, J. D. Zimmerman, C. K. Renshaw, X. Xu, R. R. Lunt, C. M. Austin, and S. R. Forrest, “Broad Spectral Response Using Carbon Nanotube/Organic Semiconductor/C60 Photodetectors”, Nano Lett., 2009, 9, 3354–3358.
- Y.-C. Tsai, J.-D. Huang, and C.-C. Chiu, “Amperometric Ethanol Biosensor Based on Poly(vinyl alcohol)–Multiwalled Carbon Nanotube–Alcohol Dehydrogenase Biocomposite”, Biosens. Bioelectron, 2007,22, 3051–3056
- Hirsch A, Angewandte Chemie-International Edition , 2002, 41,1853.
- Kuznetsova A, Popova I, Yates J. T, Bronikowski M. J, Huffman C. B, Liu J, Smalley R. E, Hwu H. H, and Chen J. G. G, Journal of the American Chemical Society, 2001, 43,10699.
- Hamon M. A, Chen J, Hu H, Chen Y. S, Itkis M. E, Rao A. M, Eklund P.C, and Haddon R. C, Advanced Materials, 1999, 10, 834.
- Hu C Y, Xu Y J, Duo S W, Zhang R F, Li M S, J Chin Chem Soc., 2009, 56, 234.
- Holzinger M, Vostrowsky O, Hirsch A, Hennrich F, Kappes M, Weiss R, AngewChem Int Ed Engl., 2001, 40, 4002.
- Jorio A, Pimenta M. A, Souza Filho A. G, Saito R, Dresselhaus G, Dresselhaus M. S, New J. Phys., 2003, 5, 139.
- Hirsch A, Angew Chem Int Ed Engl., 2002, 41, 853.
- Hamon MA, Chen J, Hu H, et al. Adv Mater., 1999, 11,8340.
- Hamon MA, Hu H, Bhowmik P, Chem Phys Lett., 2001,347–348.
- Fu, K.; Huang, W.; Lin, Y.; Riddle, L. A.; Carroll, D. L.; Sun, Defunctionalization of functionalized carbon nanotubes. Nano Lett. 2001, 1, 439-441
- Bahr, J. L.; Yang, J.; Kosynkin, D. V.; Bronikowski, M. J.; Smalley, R. E.; Tour, J. M. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: A bucky paper electrode. J. Am. Chem. Soc. 2001, 123, 653 -6542.
- Bahr, J. L.; Tour, J. M. Highly functionalized carbon nanotubes using in situ generated diazonium compounds. Chem. Mater. 2001, 13, 3823-3824.
- Pekker, S.; Salvetat, J.-P.; Jakab, E.; Bonard, J.-M.; Forro´, L. Hydrogenation of carbon nanotubes and graphite in liquid ammonia. J. Phys. Chem. B 2001, 105, 7938-7943.
- Czerw, R.; Guo, Z.; Ajayan, P. M.; Sun, Y.-P.; Carroll, D. L. Organization of polymers onto carbon nanotubes: A route to nanoscale assembly. Nano Lett. 2001, 1, 423-427.
- Fu, K.; Huang, W.; Lin, Y.; Riddle, L. A.; Carroll, D. L.; Sun, Defunctionalization of functionalized carbon nanotubes. Nano Lett. 2001, 1, 439-441.

This work is licensed under a Creative Commons Attribution 4.0 International License.









