The Use of Peanut Hull for the Adsorption of Colour from Aqueous Dye Solutions and Dye Textile Effluent
Abdunnaser M. Etorki* and Fahima M. N. Massoudi
Department of Chemistry, University of Al-Fateh, PO-Box-13203, Tripoli (Libya). Corresponding Author E-mail: abdmohet@yahoo.com
Peanut hull, an agricultural waste, proved to act as an efficient adsorbent for five anionic and cationic dyes. The process was found to be a pH dependent, suggesting the adsorption is controlled by interaction between the charged dye molecule and the surface charge on the adsorbent. The effect of variable parameters (contact time, pH, mass of adsorbent, dye concentration, agitation and activation by sulfuric acid) on the adsorption process was examined and optimum experimental conditions were established. The pH value of zero point of charge of the adsorbent was estimated and to be in the range of pH 4.5-5.5. The isothermal data for biosorption followed the Langmuir and Freundlich models and the different adsorption behaviour of basic and acidic dyes with respect to their structures is discussed.
KEYWORDS:Dye colour removal; Adsorption; Peanut hull; pH of zero point of charge; Anionic and cationic dyes
Download this article as:| Copy the following to cite this article: Etorki A. M, Massoudi F. M. N. The Use of Peanut Hull for the Adsorption of Colour from Aqueous Dye Solutions and Dye Textile Effluent. Orient J Chem 2011;27(3). |
| Copy the following to cite this URL: Etorki A. M, Massoudi F. M. N. The Use of Peanut Hull for the Adsorption of Colour from Aqueous Dye Solutions and Dye Textile Effluent. Available from: http://www.orientjchem.org/?p=11726 |
Introduction
Though the release of dyes into the environment represents only a small proportion of water pollution, but dyes are visible and so colour is the most obvious indicator for water pollution, due to their brilliance. The discharge of coloured waste from dye manufacturing, textile, pulp and paper industries besides affecting the aesthetic nature, the environmental concern with dyes is their absorption and reflection of sunlight entering the water, which interferes with the growth of bacteria to levels insufficient to biologically degrade impurities in the water1.
There are more than 100,000 commercially available dyes with over 7105 tonnes of dyes produced annually2. It is estimated that 12 % of all dyes manufactured are lost during processing and finishing operations and 20 % of this inter the environment through the municipal sewage treatment. Because of this and due to the stringent regulations laid down by the environmental authorities, colour removal from textile effluent has been the target of considerable attention.
The conventional methods for treating dyes containing wastewater are coagulation and flocculation3, ozonation4,5, membrane separation6 and adsorption7. The adsorption gave the best results as it has proved to remove different types of dyes, specially activated carbon as a preferred adsorbent for colour removal due to lot of advantage,8 but despite its intensive use remained an expensive treatment processes even on regeneration and reactivation.9
Henceforth several researchers have been studying the use of alternative materials which could be readily available at lower cost, from industrial residues or agricultural by-products. This trend was promising to achieve three points of success (i) saving non-renewable natural resources. (ii) producing valuable products with potential application in pollution control. (iii) solving the disposable problems of these industrial residues and agricultural by-products. Peanut hull has been shown to be effective adsorbent for a number of heavy metals10. Gong et. al. 11,12 reported the utilization of peanut hull as an adsorbent for anionic and cationic dyes.
Experimental methodology.
Preparation of peanut hull (adsorbent)
Peanut shells were crushed by hand, and the shells washed with water then washed with deionised water, left to dry in air then in an oven at 100°C. The dried peanut hull was ground, sieved to uniform sizes (100 – 500 µm), preserved in sealed bottles and placed in a dessiccator ready for experimental use.
Preparation of the dye adsorbate.
The five dyes used, purchased from Aldrich Chemical Company and used with no further treatment were methylene blue (MB) and crystal violet (CV) as cationic dyes. Acid green (AG), congo red (CR) and reactive blue 2 (RB) as anionic dyes. Their chemical structure and characteristic are given in Figure 1 and Table 1.
Measurement the pH of peanut hull adsorbent.
One gram peanut hull was added to 10 cm3 deionised water in a 60 cm3 glass bottle. The slurry was agitated for 3 hours and the pH of the filtrate was measured.
Infrared analysis of peanut hull.
5 mg of peanut hull was mixed with 400 mg potassium bromide powder, then pressing the mixture in a die to form a transparent pellet. The IR spectrum was recorded using an infrared spectrophotometer.
Effect of contact time on adsorption (the speed of equilibrium).
2.0 g peanut hull was added to 50 cm3 of 50 ppm solution of two dyes MB and AG in a 250 cm3 flask, shaking at a speed 300 rpm at room temperature (22°C). The contact time ranged from 15 – 240 min for acid green and 15 -180 min for methlyene blue. Filtered and the clear solution was measured photometrically.
Effect of agitation on adsorption.
The speed of agitation of the mixture of peanut hull and methylene blue dye solution was increased from 100 to 500 rpm and the clear solution was measured photometrically.
Effect of pH on the percentage removal of the dye (determination the pH of zero point of charge).
Experiments to investigate the effect of pH on the adsorption of each of the five dyes by peanut hull were performed. Dye solution (2.5 cm3 of 100 ppm) was added to a slurry of 0.2 g of peanut hull in 10 cm3 of deionised water. The pH (2 – 12.5) was adjusted using HCl or NaOH. Deionised water was added to give a total volume of 25 cm3 and a dye concentration of 10 ppm, the mixture was agitated for 10 minutes, centrifuged and filtered. The optical density of the clear solutions was measured.
Effect of mass of adsorbent on uptake.
This set of experiments was conducted by varying the concentration of the adsorbent in ten increments (from 0.1 g – 2.0 g) in 25 ml of 100 ppm methylene blue solution. The pH was adjusted to optimum values using HCl or NaOH and the final volume adjusted to 50 ml with deionised water. The concentration of the dye was determined photometrically.
Equilibrium dye adsorption isotherm.
This experiment was carried out to obtain isotherm graphs by varying the initial dye concentration of methylene blue from 50 – 600 ppm. The appropriate dye volume was added to one gram adsorbent, the pH adjusted and the volume was added to 50 cm3 with deionised water. The sample bottles were capped, sealed and shaken for one hour at room temperature (22°C). The adsorbent was separated by centrifuge and then filtered off and the dye concentration was determined photometrically.
The solution was diluted with deionised water where necessary to bring the dye absorbance within the calibration range.
Activation of the peanut hull.
A sample of the peanut hull, was washed with 3M sulfuric acid, and then washed with distilled water, until pH of the filtrate gave the same pH as distilled water. The sample was dried in an oven at 100°C overnight. The acid washed peanut hull was used to adsorb MB at different pH values and the absorbance was measured photometrically.
Measurement of colour removal using textile dyeing effluent.
0.2 g peanut hull was added to 20 cm3 of textile effluent obtained from a textile factory in TripoliLibya. Agitated and centrifuged for 10 min, filtered and the absorbance of the filtrate was measured photometrically.
All experiments were performed in duplicate to ensure reproducibility of results.
Results and discussion
The acidic nature of the adsorbent was indicated by the pH 5.7 of its slurry. Infrared spectrum suggested the presence of hydroxyl, carboxylate and amino groups. These three groups are likely to be major components in the adsorption process for the acidic and basic dyes.
The results of the contact time on adsorption, was illustrated by Plotting the percentage uptake for acid green against time, showed 85 % of the colour was adsorbed after 10 minutes reaching 97 % after 240 minutes. In the case of methylene blue almost 100 % uptake was achieved after 10 minutes, remaining constant at (100 %) after 180 minutes (Figure 2). These results confirm the strong adsorptive capacity of peanut hull for basic and acidic dyes, being slightly better for the basic dyes.
The effect of agitation on adsorption as the speed of agitation on the mixture of peanut hull and methylene blue dye solution was increased from 100 to 300 rpm, but the percentage removal decreased as the agitation increase from 300 to 500 rpm as shown in Table 2. This is to some extent due to reduction of the thickness of boundary layer; increased turbulence by agitation from 300 to 500 rpm does not result in an increased rate of adsorption.
The adsorption of dyes onto peanut hull was studied using two sets of dyes three acidic (AG, CR and RB) and two basic dyes (MB and CV). The adsorption patterns of the two sets of dyes were different; the acidic dyes had maximum adsorption at low pH and the basic at high pH (Figures 3a and 3b). This proved that the peanut hull is a good adsorbent (> 90 %) for the dyes investigated, but the maximum adsorption is pH dependent. These observations suggested the adsorption is controlled by interaction between the charged dye molecules and the surface charge on the adsorbent.
The variation of adsorption with pH of five sets of a combination of an acidic and basic dye gave the zero point of charge (Figures 4a, 4b, 4c, 4d and 4e), where the surface charge on the adsorbent changes from positive to negative and where the adsorption of the acidic and basic dye crossover in the range of pH 4.5-5.5. The use of these combinations of adsorption of negatively and positively charged dyes had shown to be a good method of obtaining the pH of zero point of charge (pHzpc) of the adsorbent as shown in Figure 5. This was also confirmed by two other methods.13
The effect of mass adsorbent on uptake, increasing the amount of peanut hull from 0.1 to 0.4 g increased the uptake to 98 %. On increasing the amount peanut hull further to 0.6 g, the uptake was again 98 %. These results showed that there was no improvement in percent adsorption by increasing the amount of peanut hull form 0.6 g to 2.0 g which indicated that 8 to 12 g dm-3 is the optimum amount of the adsorbent.
The result of the equilibrium studied is illustrated by plotting the mass of methylene blue dye in mg per mass of adsorbent in g (x/m) versus the mass of adsorbent (g) as presented in Figure 6. It shows a decrease in the values of x/m as the mass of adsorbent increases. This is due to the mass of adsorbent being a denominator in the formula used for calculating x/m values. Increasing the amount of adsorbent increases the rate of adsorption due to an increase in the number of charged site available on the surface area of adsorbent.
The result of isothermal studies (equilibrium dye adsorption isotherm) varying the initial dye concentration of methylene blue from 50 to 600 ppm. The adsorption data were analysed according to Longmuir isotherm.
Langmuir isotherm
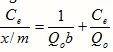
where Ce is the adsorbate equilibrium concentration in solution (mg g-1),
Q0and b are Langmuir constants related to the area occupied by a monolayer of sorbate reflecting the maximum adsorption capacity (mg g-1) and energy adsorption respectively.
x/m is the amount of dye sorbed per unit of sorbent (mg g-1).
Linear plots of Ce/x/m vs Ce showed that the adsorption followed Langmuir isotherm with good correlation coefficient (0.998) (Figure 7a). Values (51 mg of dye could be adsorbed per g of peanut hull) and b were calculated from the slope and intercept are represented in Table 3 The applicability of the Langmuir isotherm suggested the monolayer coverage of the methylene blue on the surface of peanut hull.
The use of the adsorption isotherm shape to show whether the adsorption is favourable or not, has been discussed in terms of dimensionless constant RL, referred to as separation factor14 and defined as follows:
![]()
Where b is the Longmuir constant (l/mg) and C0 the initial concentration of dye concentration (mg/l). The types of equilibrium isotherm are related with the RL values as RL> 1 for unfavourable, RL = 1 for linear, 0 < RL <1 for favourable and R= 0 for irreversible. RLvalues were found to be between zero and one (for dye concentration 50-500 ppm), Table 3 indicating the favourable adsorption of dye onto peanut hull.
The adsorption data for methylene blue were also analysed by Freundlich model as an empirical relationship describing the sorption of solutes from a liquid to a solid surface.
The logarithmic form of the Freundlich model is given by the following formula:
![]()
Where x/m is the amount adsorbed (mg/g), Ceis the equilibrium concentration of the adsorbate (mg/l), Kf and n are Freundlich constants related to the adsorption capacity and adsorption intensity. Linear plots of log x/m vs log Ce showed that the adsorption also followed Freundlich isotherm Figure 7b.
Values of Kf and n were calculated from the intercept and slope are 2.52 and 1.34 respectively with R2 = 0.997 Table 3. It has been shown using mathematical calculation that n were between 1 and 10 representing beneficial adsorption.15
Using acid-washed peanut hull adsorbent showed a higher capacity of adsorption than that achieved with water washed. A comparison of the data for water washed and acid washed peanut hull as given in Table 4. This must be a direct result of an activation process caused by the sulphuric acid washed, which can significantly increase the specific area and the porosity of the adsorbent. Similar activation processes has been reported in the literature.16-21
Adsorption by peanut hull using textile effluent gave 86 % dye removal. This proves the applicability of peanut hull adsorbent in removing the colour from aqueous dye solutions and industrial dye textile effluent.
The methylene blue showed 37 % uptake at low pH, reaching maximum uptake 96 % at pH 10.5. The crystal violet showed the start of the uptake at the low pH at 70 % and increased as the pH increased reaching 98 % uptake at pH 6.5 to 9.5. Acid green showed the maximum percentage uptake 95 % at low pH, decreasing as the pH increased reaching zero uptakes at pH 10.5. Similarly, the reactive blue 2 showed maximum percentage uptake 91 % at low pH but decreasing to zero uptake at pH more than 9.5. Congo red showed maximum percentage uptake 96 % at low pH and decreasing to 50 % uptake at pH 12.5.
An explanation of the low adsorption of methylene blue at low pH (37 %) compared to crystal violet (70 %) is possibly due to the lesser number of N,N-dimethyl groups (two in MB compared to three in CV), the two methyl electron releasing groups making their nitrogen atoms less positive . This also attributed to the formation of surface hydrogen bonding between the hydroxyl groups on the adsorbent surface and the two nitrogen atoms of methylene blue and the three nitrogen atoms of crystal violet, as illustrated in Figure 8.
The ionic nature of the two basic dyes (methylene blue and crystal violet) could have played a role in retaining the dye species on the surface of the adsorbent. The molecular structure of methylene blue has ionic charge, with decentralised or delocalised positive charge on its organic structure as shown in Figure 9. Similar arguments could be applied to the structure of crystal violet.
On the other hand, the acidic dyes (acid green, reactive blue 2 and congo red) are an ionic dyes. The presence and the number of anions groups (SO32-) on the dye molecule is an important factor in their different adsorptive behaviour. For congo red the uptake of colour was rapid 97 % at pH 2, decreased to 50 % at pH 12.5. Similarly the uptake of acid green and reactive blue 2 is 95 % and 91 % respectively at pH 2, deceases as the pH increase, reaching zero uptakes at pH 11.
In an acidic medium, the surface of the adsorbent will be slightly protonated and favour the uptake of anionic dyes. When the pH increase from 2 – 12.5 the degree of protonation on the adsorbent surface is reduced gradually and hence decreased in adsorption was observed.
 |
Figure 1: Chemical structure of the dyes used Click here to View Figure |
Table 1: The characteristics of the dyes used
|
Dyes |
Molecular weight |
Wavelength (nm) |
CI |
Type |
|
Acid green |
622.56 |
642 |
42095 |
Anionic |
|
Congo red |
696.67 |
497 |
22120 |
Anionic |
|
Crystal violet |
407.99 |
588 |
42555 |
Cationic |
|
Methylene blue |
319.86 |
665 |
52015 |
Cationic |
|
Reactive blue 2 |
840.12 |
607 |
61211 |
Anionic |
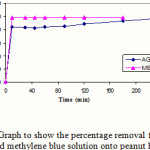 |
Figure 2: Graph to show the percentage removal for 50 ppm acid green and methylene blue solution onto peanut hull adsorbent |
Table 2: Percentage uptake with increase of agitation speed
|
Agitation speed (rpm) |
% uptake |
|
100 |
80 |
|
200 |
93 |
|
300 |
97 |
|
400 |
90 |
|
500 |
88 |
 |
Figure 3 Click here to View Figure |
 |
Figure 4 Click here to View Figure |
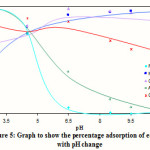 |
Figure 5 Click here to View Figure |
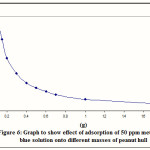 |
Figure 6 Click here to View Figure |
Table 3: Longmuir and Freundlich isotherms constant
| Dye | Langmuir constants | Correlation coefficient | Freundlich constant | Correlation coefficient | |||
|
Methylene blue |
Qe mg/g |
b (l/mg) |
RL |
R2 |
Kf |
n |
R2 |
|
51.02 |
0.0414 |
0.326-0.046 |
0.998 |
2.52 |
1.34 |
0.997 |
|
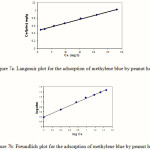 |
Figure 7 |
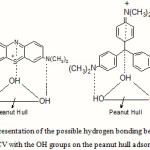 |
Figure 8 Click here to View Figure |
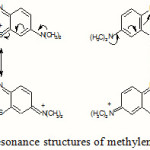 |
Figure 9 Click here to View Figure |
Table 4: The percentage uptake for water washed and acid washed peanut hull adsorbent in the pH range 2 to 12.5
|
pH |
% uptake (water washed) |
% uptake (acid washed) |
|
|
2 |
37 |
71 |
|
|
4.5 |
81 |
98 |
|
|
6.5 |
92 |
99 |
|
|
8.5 |
95 |
99 |
|
|
9.5 |
96 |
99 |
|
|
10.5 |
96 |
98 |
|
|
12.5 |
87 |
95 |
|
Conclusion
Peanut hull was proved successful as a cheap, locally available, renewable adsorbent for the removal of colour of aqueous solution of dyes and textile dyeing effluent. It was proved to be a good adsorbent (> 90 %) for the dyes studied and the process was found to be pH dependent.
The adsorption capacity of the adsorbent was investigated using two sets of dyes, three acidic (AG, CR and RB) and two basic (MB and CV). The adsorption pattern of the two sets of dyes was different, suggesting the adsorption was controlled by interaction between the charged dye molecules and the surface charge on the peanut hull.
The variation of adsorption with pH of five sets of combination of an acidic and basic dye was shown to be a good method for obtaining the pH value of the zero point of charge of the peanut hull. The strong adsorptive capacity of the peanut hull for both basic and acidic dyes (being better for the basic dyes) was demonstrated by the result of the contact time experiment.
The result of the equilibrium studies showed an increase in the percentage uptake of methylene blue as the concentration of the adsorbent increase, indicating a decrease in the value of x/m with the increase of adsorbent mass. In the adsorption isotherm experiment, increasing the dye concentration of methylene blue, as the dye molecule was adsorbed onto the adsorbent surface and fewer sites became available the adsorption decreases. This deceasing removal rate suggested the formation of a monolayer of dye molecules on the outer surface of the adsorbent.
The Longmuir and Freundlich isotherms have given a straight line in both cases with regression values of 0.998 and 0.997 respectively.
The effect of increase agitation on adsorption showed an increase in the percentage removal of the dye, similarly washing the peanut hull with sulphuric acid. Using a dyeing textile effluent resulted in 86 % colour removal.
the low adsorption of methylene blue at low pH (37 %) compare to crystal violet (70 %) was explained with the lesser number of N,N-dimethyl groups (two in MB and three in CV) and was also attributed to the formation of surface hydrogen bonding between the hydroxyl group on the adsorbent surface and the two nitrogen atoms of methylene blue and the three nitrogen atoms of crystal violet.
References
- Pierce.J, J. Soc. Dyers Colour, 110, 131 (1994).
- Seshadri.S,Bishop.P.L and Agha.A.M, Waste Management, 15, 127 (1994).
- Poots.P and McKay.M, Water Research, 10, 1076 (1979).
- Ghosh.M.M,Woodard.F.E, Sproul.O.J, Knowlton.P.H and Guertin.P.D, J. Wat. Poll. Cont. Fed., 50, 1776 (1978)..
- Horning.R.H, Textile Chem. Colour, 9, 24 (1977)..
- Keith.S,“Electrochemical Process for Clean Technology”, The Royal Society of Chemistry, UK, (1989).
- Rattee.I.D and Breuer.M.M “The Physical Chemistry of Dye Adsorption”, Academic Press, Inc. London, New York, (1974).
- Bansal.R.C, Dounet.J.B and Stockli.F, “Active Carbon”, Marcel Dekker, New York, (1988).
- Kinoshita.K, “Carbon Electrochemical and Physicochemical Properties”, John Wiley & Sons Inc., (1988)..
- Namasivaym.N and Periasamy.K, Ind. Eng. Chem. Res., 33, 317 (1994)..
- Gong.R,.Li.M, Yang.C,. Sun.Y and J. Chen, J. Hazardous Materials, 121, 247 (2005).
- Gong.R,Li.M,Yang.C, Sun.Y and Chen.J,Dyes and Pigments,64, 187 (2005)..
- Noh.J.S and Schwarz.J.A, J. Coll. Interf. Sci., 1989, 130, 157 (1989).
- Treyball, “Mass Transfer Operations”, 3rd ed., New York, McGraw Hill, (1980).
- McKay.G, J. Chem. Technol. Biotechnol., 32, 759 (1982).
- Bonelli.P.R, Della.P.A. Rocca.R, Cerrella.E.G and Cukierman.A.L, Bioresource Technol., 76, 15 (2001).
- Girgis.B.S, Yanis.S.S and Soliman.A.M, Mat. Lett., 57, 164 (2002).
- Jagtogen.M and Derbyshire.F, Carbon, 1998, 36, 1085 (1998)..
- Benaddi.H, Bandosz.T.J, Schwarz.J.A, Jagiello.J, Rouzaud.J.N,Legras.D and Beguin.F, Carbon, 38, 669 (2000).
- Namasivaym.N, and Periasamy.K Sep. Sci. Technol, 30, 2223 (1995).
- Dastgheib.S.A and Rockstraw.D.A, Carbon, 40, 1853 (2002)..

This work is licensed under a Creative Commons Attribution 4.0 International License.









