Synthesis of Graft Biocopolymer Based on Pectin and Investigation of Chemorheology Properties
Mohammad Sadeghi1* and Mojgan Yarahmadi2
1Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak Iran. 2Department of English, Humaniti Faculty, Islamic Azad University, Arak Branch, Arak Iran.
In the present paper, synthesis and investigation of the rheological behavior of a graft copolymer based on Pectin (Pec) and polyacrylonitrile (PAN) was carried out . The physical mixture of Pec and PAN was hydrolyzed by NaOH solution to yield Pec-poly(sodium acrylate-co-acrylamide) superabsorbent hydrogel. The nitrile groups of PAN were completely converted to a mixture of hydrophilic carboxamide and carboxylate groups during alkaline hydrolysis followed by in situ crosslinking of the PAN chains by the alkoxide ions of Pectin. A proposed mechanism for hydrogel formation was suggested and the hydrogel structures were confirmed by FTIR spectroscopy, SEM and porosimeter. The rheological behavior of the prepared gels were measured with rheometer.
KEYWORDS:Pectin; polyacrylonitrile; hydrogel; chemorheoloy; rheology
Download this article as:| Copy the following to cite this article: Sadeghi M, Yarahmadi M. Synthesis of Graft Biocopolymer Based on Pectin and Investigation of Chemorheology Properties. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Sadeghi M, Yarahmadi M. Synthesis of Graft Biocopolymer Based on Pectin and Investigation of Chemorheology Properties. Orient J Chem 2011;27(2). Available from: http://www.orientjchem.org/?p=24824 |
Introduction
The modification of natural polymers is a promising method for the preparation of new materials. Graft copolymerization of vinyl monomers onto natural polymers is an efficient approach to achieve these materials. Superabsorbing resins were first developed with a view to utilizing agricultural materials, and are typed by the hydrolyzed starch-g-poly(acrylonitrile), H-SPAN (1). Since then, starches from different resources as well as other polysaccharides, for example, cellulose, hydroxyethyl cellulose, agar , sodium alginate and guar gum were graft copolymerized to achieve water absorbing polymers. Polyacrylonitrile (PAN), polyacryamide, and poly(acrylic acid) have been frequently grafted, mostly onto starch, using different initiators especially the ceric-saccharide redox system (2). Radical polymerization, however, has several disadvantages. The reproducibility of this method is poor, and there is little control over the grafting process, so the molecular weight distribution is polydisperse. In addition, the necessity for inert gases (e.g., argon) to prepare an oxygen-free atmosphere and the need for initiators, toxic and/or expensive monomers, and crosslinkers are other disadvantages of free-radical polymerization reactions. These problems have been reviewed in detail (3). For the first time, Fanta et al. (4), with a new method, tried to synthesize of HSPAN superabsorbent hydrogel. They indicated by a solubility test that crosslinks were formed during graft copolymerization, by coupling of the two growing PAN radicals, and during saponification, by the attack of Pectin alkoxide ions on the nitrile groups as the initioation reaction of nitrile polymerization in the early stages of saponification. The nitrile groups of PAN were converted to a mixture of hydrophilic carboxamide and carboxylate groups during alkaline hydrolysis followed by in situ crosslinking of the grafted PAN chains. The initially formed oxygen–carbon bonds between the Pectin hydroxyls and nitrile groups of the PAN chains remained crosslinking sites. Then, Fanta and Doane (4) attempted to extend this idea to the preparation of superabsorbent hydrogels by the saponification of PAN in the presence of polyhydroxy polymers. Finally, Yamaguchi et al.1(5) reported the preparation of superabsorbing polymers from mixtures of PAN and various saccharides or alcohols.
Also,The chemical reaction has a pronounced effect on the molecular structure of polymers. In addition, rheological properties of polymers depend on the molecular mobility. Consequently, it is possible to monitor rheological properties of a reacting system ,i.e., viscosity or modulus (storage and loss modulus) by using a chemorheological approach due to following the reaction. Chemorheology is a powerful tool to study chemical cross-linking reactions at which a transition from the liquid to the solid state takes place. A reactive thermosetting resin can be used in an industrial production process with respect to its curing performance. The question of interest for a thermosetting polymer is determination of its gelation temperature and gelation time. These points of gelation are the basic parameters characterizing processability of a thermosetting resin (13,14). Indeed, In this investigation, we paid attention to the synthesis and investigation of the rheological behavior graft copolymer based on Pectin and PAN.
Experimental
Materials
Pectin (chemical grade, MW 50000) was purchased from Merck Chemical Co. (Germany). Polyacrylonitrile (PAN) was synthesized through a method mentioned in the literature(6). Double distilled water was used for the hydrogel preparation and swelling measurements.
Hydrogel preparation
A facial one step preparative method was used for synthesis of Pec-poly(sodium acrylate-co-acrylamide) hydrogel, Pec-poly(NaAA-co-AAm), hydrogel. A general procedure for alkaline hydrolysis of Pec-PAN mixture was conducted as follows. Pectin(0.50-1.33 g) was added to a three-neck reactor equipped with a mechanical stirrer (Heidolph RZR 2021, three blade propeller type, 50-500 rpm), including 35 mL doubly distilled water. The reactor was immersed in a thermostated water bath. After complete dissolution of Pectin to form a homogeneous solution, certain of sodium hydroxide (0/25-2/5N) was added to the Pectin solution at desired temperature (alkalization temperature, 20-120 oC). The mixture was allowed to stir for certain times (alkalization times, 20-90 min). The various amount of polyacrylonitrile (0.50-4.50 g) was dispersed in the reaction mixture to saponify for certain times and temperatures (alkaline time and temperature). During the saponification NH3 gas was evolved and a color change from red to light yellow. This discoloration was an indication of the reaction completion. The pasty mixture was allowed to cool to room temperature and neutralized to pH 8.0 by addition of 10 wt % aqueous acetic acid solution. Then the gelled product was scissored to small pieces and poured in ethanol (200 mL) to dewater for 5 h. The hardened particles were filtered and dried in oven (50 oC, 10 h). After grinding, the powdered superabsorbent hydrogel was stored away from moisture, heat and light.
Results and Discussion
Mechanism of hydrogel formation
A general reaction mechanism for Pec-poly(AN) hydrogel formation is shown in Scheme 1. At the first step, hydroxyl groups of Pectin substrate was converted to corresponding alkoxide ions using sodium hydroxide solution (Scheme 1). Then, these macroalkoxides initiate crosslinking reaction between some adjacent polyacrylonitrile pendant chains. This reaction leads to intermediate formation of naphthyridine cyclic structures (including imine, -C=N-, conjugated bonds) with deep red color. The intermediate was then hydrolyzed using residual sodium hydroxide aqueous solution to produce hydrophilic carboxamide and carboxylate groups (Scheme 1) with a resulting color change from red to light yellow. This sharp color change was used as an indication to halt the alkaline treatment. However, incompletely hydrolyzed structures may also give rise to a few crosslinking points result in a loosely crosslinked network. It has been reported, in the case of H-SPAN, a maximum conversion of 70% of nitrile to carboxyl groups and the remaining 30% are amide groups (7-8).
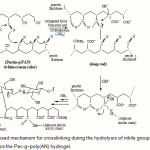 |
Scheme 1: Proposed mechanism for crosslinking during the hydrolysis of nitrile groups of the Pec–PAN mixture to produce the Pec-g–poly(AN) hydrogel. |
In fact, details of the chemical processes and mechanism involved in H-SPAN synthesis are not yet well understood. For instance, the incomplete hydrolysis is interpreted as being related to steric and polar factors(9). Weaver et al. suggested that condensation might also occur between carboxyl and amide groups to form imide structures(10). Therefore, in the case of our hydrogel, Pec-poly(NaAA-co-AAm), we realized that precise control of the ratio is practically impossible.
Infrared spectroscopy was carried out to confirm the chemical structure of the hydrogel. Figure 1 shows the FTIR spectra of Pec-PAN physical mixture and the resulted hydrogel, Pec-poly(NaAA-co-AAm). The band observed at 2242 cm-1 can be attributed to stretching of –CN group of polyacrylonitrile (Fig. 1b). The hydrogel comprise an Pectin backbone with side chains that carry carboxamide and carboxylate functional groups that are evidenced by three new peaks at 1407, 1556, and 1675 cm-1 (Fig. 1c). These peaks attributed to C=O stretching in carboxamide functional groups and symmetric and asymmetric stretching modes of carboxylate groups, respectively(11). The stretching band of –NH overlapped with the -OH stretching band of the Pectin portion of the copolymer. As shown in Fig. 1c and Scheme 1, after alkaline hydrolysis, most of the nitrile groups are converted to carboxamide and carboxylate groups.
To obtain an additional evidence of in situ crosslinking during alkaline hydrolysis, a similar reaction was conducted in absence of the polysaccharide. Since the resulted product became soluble, the crosslinks really formed between the alkoxide ions of Pectin and the nitrile groups of PAN. This fact practically proves that the starch hydroxyls are involved in the crosslinking.
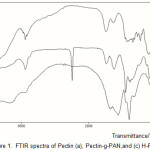 |
Figure 1: FTIR spectra of Pectin (a),Pectin-g-PAN,and (c) H-Pectin-g-PAN Click here to View table |
Scanning electron microscopy
One of the most important properties that must be considered is hydrogel microstructure morphologies. The surface morphology of the samples was investigated by scanning electron microscopy. Figure 2 shows an SEM micrograph of the polymeric hydrogels obtained from the fracture surface. The hydrogel has a porous structure. It is supposed that these pores are the regions of water permeation and interaction sites of external stimuli with the hydrophilic groups of the graft copolymers.
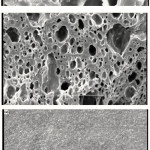 |
Figure 2: SEM photograph of the Pectin(a), and hydrogel Surfaces with scale bar 10 and 50 μm(b,c). |
Rheological Properties of hydrogel
The rheological test carried out with UDS 200 AntoanParr bob and cup rheometer. On the contrary, dynamic oscillatory measurements allow accurate determination of the gel time of thermosetting systems. In these experiments, the evolution of the storage modulus (G’) and the loss modulus (G”) is measured in small amplitude oscillatory shear as a function of cross-linking time while, frequency is kept constant throughout(13). As an example, a plot of the G’ and G” versus time at 120°C is presented in Figure 3. Trends in the changes of storage and loss moduli at different isothermal temperatures are the same.
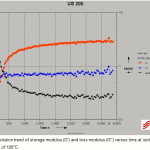 |
Figure 3: Evolution trend of storage modulus (G’) and loss modulus (G”) versus time at isothermal temperature of 120°C. Click here to View figure |
Rheological properties such as G’ and G” are very sensitive to the variation of the molecular structure and phase transitions occurring in thermosetting polymer systems(14).Cross-linking of thermosetting polymers can be modeled as a cluster formation process(15). During the initial period of reaction, micro-gels are formed with branched and partially cross-linked molecules of colloidal sizes(16). The polymer continues to react to form larger clusters of various sizes distributed randomly in the system(17).
Rheologically, the thermosetting resin in the early stage of curing is in liquid state and viscous behavior dominates the initial part of the curing process, subsequently G”>G’. Both of the dynamic moduli increase as a result of increasing cross-link density and molecular weight of curing polymer system. at the gel point an infinitely large cluster extends throughout the whole system, and a three-dimensional continuous network is formed and a crossover of G’ and G” curves occurs (14). The cross-over point of G’ and G” during thermoset curing can be applied as a criterion for elasticity domination in a reactive system and has been considered as the gel point (18).
The loss factor is high at the beginning of the test and decrease with time (Fig. 4). After the initial scatter and increase of the loss factor, at a later reaction stage, tan () starts to decrease as the elastic part of the complex modulus (G’) operates because of the formation of elastically active cross-links before the gel point(14).
Figure 4 shows the viscosity change during isothermal curing of the gelatin reactive formulation at the strain amplitude of 1% and dynamic frequency of 10 s-1. As it can be seen in Figure 3, at isothermal curing temperature is observed a steep increase of the value of complex viscosity, reflecting a phase transition from liquid to solid. In some reports, the gel time has been determined as the time when the viscosity of the reacting system tends to infinity (19).
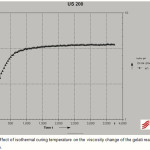 |
Figure 4: Effect of isothermal curing temperature on the viscosity change of the gelati reactive formulation. Click here to View figure |
Effect of shear thinning for Gelatin reactive formulation is showed in fig. 5. It is show at low shear rates the viscosity increase and with increasing of the shear rate the viscosity decrease. By progression of the reaction, high molecular weight polymer chains are formed. Consequently, the application of shear rate can induce a shear thinning behavior in long chain entangled macromolecules
Application of the shear rate causes orientation of molecular chains which leads to shear thinning behavior and the dilution effect of shear rate on viscosity (shear thinning behavior) seems to be dominant. Hence, the viscosity of the reactive formulation at higher shear rates is lower than that in lower shear rates (20).
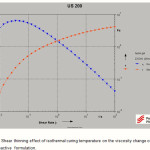 |
Figure 5: Shear thinning effect of isothermal curing temperature on the viscosity change of the gelatin reactive formulation. Click here to View figure |
Conclusion
The superabsorbent hydrogel, Pec-poly(AN), was synthesized through alkaline hydrolysis of Pectin-PAN physical mixture. The reaction of Pectin alkoxide anions with nitrile groups of polyacrylonitrile, forms crosslinking points and results in a three-dimensional network. Because a polymerization reaction is not involved, so there is no need to initiator, toxic and/or expensive monomer and crosslinker. Therefore, problems such as polymerization control, conversion loss, and residual monomer are eliminated. Indeed, since no toxics material is used for the synthesis, this practical approach may be preferred to as a relatively “green process”. In addition, this one-step preparative method conducted under normal atmospheric conditions in a short period of time. The dark red-yellow color change provides a visual indication for recognizing the reaction completion.
In this study, the rheometry results showed that trend of the change in gelation times lower temperature sensitivity above the gelation temperature.
By progression of the reaction and formation of long chain macromolecules shear thinning behavior was observed in viscoelastic regions. The shear thinning phenomenon of long chain gelatin molecules counters the influence of the shear rate on the increase of the mobility of functional groups. The evolution of the storage modulus (G’) and the loss modulus (G”) is measured in small amplitude oscillatory shear as a function of cross-linking time while, frequency is kept constant throughout. The gelation time was obtained from the modulus’s cross over.
References
- Buchholz FL, Graham AT. Modern Superabsorbent Polymer Technology. Elsevier: Amsterdam.(1997).
- Peppas, LB.; Harland, RS. In Absorbent Polymer Technology; Elsevier: Amsterdam.(1990).
- Po, R. J Macromol Sci-Rev Macromol Chem Phys, C34, 607.(1994).
- Fanta, G. F.; Burr, R. C.; Doane, M. W. ACS Symp Ser, 187, 195(1982).
- Yamaguchi, M.; Watamoto, H.; Sakamoto, M. Carbohydr Polym, 9, 15(1988).
- Dorkoosh, FA.; Brussee, J.; Verhoef, JC.; Borchard, G. Rafeiee-Tehrani, M.; Juninger, HE. Polymer, 41, 8213.(2000).
- Raju, KM.; Raju, MP.; Mohan, YM. J Appl Polym Sci, 85, 1795.(2002).
- Lim, DW.; Yoon, KJ.; Ko, SW. J Appl Polym Sci, 78, 2525.(2000).
- A. M. Mathur, S. K. Moorjani, A. B. Scranton. Macromol. Sci.-Rev. Macromol. Chem. Phys., C36 ,405.(1996).
- A. Suzuki, T. Ishii, Y. Maruyama, J. Appl. Phys., 80 ,131–136(1996).
- A. Mamada, T. Tanaka, D. Kungwachakun, M. Irie, Macromolecules, 23,1517–1519(1990).
- Einerson NJ, Stevens KR, Kao WJ. Biomaterials, 24, 509(2003)
- Winter H.H. Can the gel point of a cross-linking polymer be detected by the G’-G” cross over?, Polym. Eng. Sci., 27, 1698-1702(1987).
- Cai J.J., Salovey R. Chemorheology of model filled rubber compounds during curing, Polym. Eng. Sci., 41, 1853-1858(2001).
- Muthukumar.M. Screening effect on viscoelasticity near the gel point, Macromolecules, 22, 4656-4658(1989).
- Malkin A.Y., Kulichikhin S.G. Rheokinetics of curing, Adv. Polym. Sci., 101, 217-257(1991).
- Muzumdar S.V., Lee J.L.Chemorheological analysis of unsaturated polyester-styrene copolymerization, Polym. Eng. Sci., 36, 943-953(1996).
- Dean D., Walker R., Theodore M., Hampton E., Nyairo E.Chemorheology and properties of epoxy/layered silicate nanocomposites, Polymer, 46, 3014-3021(2005).
- Hong B.T., Roh S.S., Kim D.S. Chemorheologicalstudies on a dicyanate resin modified with poly(ether sulfone), Polym. Int., 53, 640-645(2004).
- Dealy J.M. Melt Rheology and Its Role in Plastic Processing, Van Nostrand and Reinhold, New York, Ch.12.(1990).

This work is licensed under a Creative Commons Attribution 4.0 International License.









