Measurement of Critical Micelle Concentration of Technical Grade Non-Ionic Detergent In Presence of Chloramine-T using Dye Solubilization Technique
A. A. Patil and T. J. Patil
Department of Chemistry, Z.B.Patil College, Deopur, Dhule - 424 002, India.
Commercially available technical grade non-ionic surfactants usually contain impurities and have a broad distribution of molecular weight owing to degree of ethoxylation. It has shown that the surface tension method (Wilhelmy plate) is very sensitive if impurities are present in surfactant. Much lower CMC values are obtained for technical grade surfactants with the surface tension method than with the dye solubilization method. The CMC determined by dye solubilization technique are in fair agreement with the values obtained by other available methods. The change in critical micellar concentration of Tween-40 has been studied through the influence of additive Chloramine-T in aqueous medium by measuring the absorbance of the pure surfactant and with Chloramine-T in presence of water insoluble dye Orange-OT by using dye solubilization technique. The absorbance was found to be increased with increased concentration of pure surfactant Tween-40. The absorbance of the mixed systems with Chloramine-T also shows the same trend. The CMC of pure surfactant get decreased with increased concentration of additive Chloramine-T. The influence of additive Chloramine-T on the absorbance of Tween-40 is clear indication that the phenomenon of dye micellization is associated with the different micelles coalescing.
KEYWORDS:Dye Micellization; Dye Solubilization; Absorbance (A); Tween-40 (TW-40); Chloramine-T (CAT); Critical Micelle Concentration (CMC)
Download this article as:| Copy the following to cite this article: Patil A. A, Patil T. J. Measurement of Critical Micelle Concentration of Technical Grade Non-Ionic Detergent In Presence of Chloramine-T using Dye Solubilization Technique. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Patil A. A, Patil T. J. Measurement of Critical Micelle Concentration of Technical Grade Non-Ionic Detergent In Presence of Chloramine-T using Dye Solubilization Technique. Available from: http://www.orientjchem.org/?p=24717 |
Introduction
The critical micelle concentration of technical grade non-ionic surfactants has been determined in the past by three different methods1, viz., from the break in the turbidity Vs. concentration curve, from the break in the static surface tension Vs. logarithm of concentration curve and by the iodine solubilization technique2. Thus CMC determinations are usually on the basis of sharp change in the colligative properties like clouding, surface tension, solubilization of a hydrophobic dye (Orange-OT).
Ionic surfactants are easier to obtain in the pure form than ethoxylated non-ionic surfactants. Commercially available technical grade ethoxylated surfactants have a istribution in the degree of ethoxylation as well as in the hydrophobe. For non-ionic surfactants large variation is observed in the CMC values determined by different methods. Also for non-ionic surfactants a clear break in the surface tension Vs. concentration curve is not usually obtained due to presence of impurities and a broad molecular weight distribution3,4. CMC’s of surfactants can be determined by number of methods5,6
The CMC of non-ionic detergents are much less than those ionic detergents of comparable hydrocarbon chain length because of which the time required to reach equilibrium in each measurements of surface tension can be several hours7. The surface tension method thus becomes not only time consuming but may involve uncertainties8 due to evaporation from the surface and stagnant layer formation9. The experimental difficulties involved in the precision determination of clouding are well known10, though not insurmountable. The iodine solubilization technique is convenient but presumably involves chemical reaction leading to the formation of hydrogen iodides11 and therefore cannot be considered to be reliable as compared to dye solubilization method. The solubilizing power is one of the most important properties of surfactants. The solubilization of water insoluble dye Orange-OT in the surfactant micelles was studied in order to determine CMC of the surfactants. The amount of the dye solubilized was insignificant up to the CMC of each surfactant or with additive and thereafter a sudden steep rise was observed with the formation of micelles in the bulk.
Polysorbates are a class of emulsifiers used in some pharmaceuticals and food preparations. They are often used in cosmetics to solubilize essential oils in to water based products. Tween-40 (TW-40) is one of the polysorbate. TW-40 is nonionic detergent used for cell lysis, nuclei isolation and cell fractionation. It is also widely used in cosmetics and some pharmaceutical preparations. It has been shown earlier that CMC of ionic or non-ionic surfactants get affected (decreased) by the addition of salts12. Some inorganic and organic compounds are added to detergents in order to make detergent cheap, user friendly and to boost its power13, these compounds are called as “builders”. Chloramine-T (CAT) is used as disinfectant, algaecide, bactericide, germicide for parasite control and for drinking water disinfection. Due to these properties CAT can be used as a “builder” in detergents.
The present paper reports the effect of Chloramine-T (CAT) on CMC of pure non-ionic surfactant Tween-40 at various concentrations of CAT by using dye solubillization technique. These studies are important in the field of medicinal preparations, agrochemicals, detergents etc.
Materials and Methods
The non-ionic surfactant Tween-40 (M.Wt.1283.65) and Chloramine-T trihydrate (M.Wt.281.69) were the products of Sigma-Aldrich, USA and these were used as received. The dye Orange-OT (1-o-tolyl azo- 2-napthol, M.Wt.262.3) prepared from o-toluidine and 2-napthol was purified twice by precipitating it from acetone solution with water followed by recrystallization from ethyl alcohol. Doubly distilled water with specific conductance 2-4 μScm-1 at 303.15K is used in the preparation of all solutions of different concentrations.
Dye Solubilization
The insoluble dye Orange-OT was shaken with an aqueous solution of the surfactant for 48 hours at room temperature by using mechanical stirrer and then the residue was removed by means of centrifugation and filtration. The absorbance of the resultant solution was measured by using Eqiptronics Digital Spectrophotometer Model: EQ-820 at λmax = 470nm and at 303.15K
 |
Scheme 1 Click here to View Scheme |
The absorbance for pure surfactant increases with increase in concentration for surfactant Tween-40. The absorbance values of surfactant solutions at 470nm wavelength are plotted as a function of surfactant concentration in weight percentage to measure the extent of dye uptake (Fig.1)
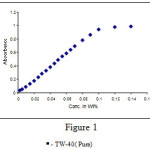 |
Figure 1 Click here to View Figure |
 |
Figure 2 Click here to View Figure |
Below the CMC, the rise in absorbance is small, where as above the CMC, the rise in absorbance is sharp. For non-ionic surfactant the micellization process is known to be less sharp than for ionic surfactant hence the rise in absorbance varies strongly over a range of surfactant concentration. The absorbance Vs concentration curve get flattened at high enough surfactant concentration as most of the dye forms micelles, depleting the continuous-phase dye. The linear portion near the inflection point is extrapolated to the point where the absorbance matches that of the dye in the absence of any surfactant. This concentration is defined as the CMC. The observed CMC value for pure surfactant Tween-40 was 0.0241 which is very close to the reported CMC value 0.02714.
Tween-40 and Chloramine-T systems:
The absorbance for mixed system also increases slowly with increase in concentration up to CMC and then increases sharply with increase in concentration above CMC. The absorbance values of mixed systems of Tween-40 and Chloramine-T at λmax = 470nm wavelength are plotted as a function of surfactant concentration in weight percentage to measure the extent of dye up take (Fig.2)
Surfactant Tween-40 in presence of additive Chloramine-T shows that the amount of dye solubilized rise slowly up to the CMC, thereafter a sudden and sharp rise was observed with the formation of micelles in the bulk. The CMC Tween-40 surfactant was found to be decrease with increase in the concentration of added solute Chloramine-T. Thus the solubilizing power of surfactants increases in the presence of Chloramine-T. The CMC values obtained for mixed systems are shown in Table 1
Table 1: Influence of [CAT] on CMC of detergent at λmax = 470nm
|
Detergent with [CAT] wt% |
CMC(mM) of Detergent at 303.15K |
| 0.0001 | 0.0239 |
| 0.001 | 0.0179 |
Acknowledgement
The author (A. A. Patil) is thankful to Dr. A.Z. Patil, Hon’ble Chairman, JET’s Z.B.Patil College, Dhule, Hon’ble Principal; Z.B.Patil College, Dhule, Head, Department of Chemistry and all colleagues of Department of Chemistry, Z.B.Patil College, Dhule for their kind co-operation.
References
- P. Becher in “Non-ionic Surfactants” M. J. Schick, Ed., Marcel Dekker, New York, N.Y., p478,1967.
- S. Ross and J. P. Olivier, J. Phys. Chem., 63, 1671(1959)
- Alexandridis P. , V. Athanassiou, S. Fukuda and T. A. Hatton, Langmuir, 10, 2604(1994)
- Mysels K. J. , and R. E. Stafford, Colloids Surf., 51,105(1990)
- Shinoda K. , and T. Nakagawa, Colloidal Surfactants : Some Physicochemical Properties, Academic Press, New York, 1963
- Hunter R. J., Foundations of Colloidal Science, Vol.1, OxfordUniversity Press, Oxford, 1987.
- P. H. Elworthy and C. B. MacFarlane, J. pharm. Pharmacol., 14, 100T(1962)
- P. Mukerjee, Advan. Colloid Interface Sci., 1, 241(1967)
- J. T. Davies and E. K. Rideal, “ Interfacial Phenomena” Academic Press, New York, N.Y., 1961
- P. H. Elworthy and C. B. MacFarlane, J. Chem. Soc. , 537, (1962)
- T. Nakagawa in “Non-ionic Surfactants” M. J. Schick, Ed., Marcel Dekker, New York, N.Y., 588 (1967)
- Jay Patel, Dharmesh Varade and Pratap Bahadur, Ind. J. of Chem., 43A, 715(2004)
- P. G. T. Fogg, J. Chem . Soc., 83, 117(1958)
- A. Patist, S.S. Bhagwat, K.W. Penfield, P. Aikens, and D.O. Shah, Journal of Surfactants and Detergents, 3, 53(2000)

This work is licensed under a Creative Commons Attribution 4.0 International License.









