Debromination of Aurone Dibromide with Sodium Hydrogen Sulphide
S.K. Doifode, M.P. Wadekar and Suresh Rewatkar
Department of Chemistry, Government Engineering College, Amravati - 444 604, India.
Debromination; Aurone Dibromide; Sodium Hydrogen Sulphide
Download this article as:| Copy the following to cite this article: Doifode S. K, Wadekar M. P, Rewatkar S. Debromination of Aurone Dibromide with Sodium Hydrogen Sulphide. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Doifode S. K, Wadekar M. P, Rewatkar S. Debromination of Aurone Dibromide with Sodium Hydrogen Sulphide. Available from: http://www.orientjchem.org/?p=24662 |
Introduction
Debromination of Vicinal-dihaloakanes and dibromoketones have been studied by earlier workers using the debrominating agents like potassium iodide in acetone1, stannous chloride in different solvent2, sodium hydrogen selenide3, thiourea in ethanol4,5 and also by some workers using hydrated sodium sulphide under phase transfer condition6,7 Photochemical and chemical reduction of vicinal dihalides via phase transfer of 4,41 bipyridinium radical8 for solar energy conversion, use of cobalt octacarbonyl on alumina have been reported for selective dehalogenation of <-bromo sulphoxides9. Chromium (II) acetate also brings reductive debromination in vicinal dihaloalkanes10and chalcone dibromide11. Sodium sulphide with vicinal dibromo derivative of certain oximes is reported to give sulphoxide derivatives.12 Chalcone with anhydrous sodium sulphide in acetyl acetone at room temperature is reported to give (Ph-CO -CH2-CO -Ph) Thiourea with 2-hydroxy Chalcones gives 1,3 thiamine derivatives in alkaline ethanol on refluxing for 3 hours while in 10 min in DMSO.14 Sodium polysulphide with chalcone is reported to form 2,4-dibenzoyl -3,5-diphenyl thiolanes15.
Recently sodium hydrogen sulphide reacts with chalcone dibromide and stibene dibromide in methanol to give chalcone and stibene respectively16.
It was thought interesting to use sodium hydrogen sulphide for debromination of aurone dibromide in methanol.
Aurone dibromide (0.01 mole) was treated with freshly prepared sodium hydrogen sulphide (0.02 mole) in dry methanol (30 ml.). The reaction mixture was heated for 20 mins, diluted with water and the product was crystallized form ethanol. The product was found to be identical with aurone on the basis of m.p., m.m.p. (mixed melting point) and co-TLC and spectral data of uv-vis, IR and NMR 2,α (4’ methoxy benzyl)coumaran -3- one mp 158 0C.It is white powdery solid compound having MP-1580C. It does not give any colouration with nautral ferric chloride solution. From the analytical data, the molecular formula was found to be C17 H14 Br2 03. The molecular wt is 426
TLC
Rf value was found to be 0.36 for benzene as a solvent on silica gel G plate with a layer thickness of 0.3 mm. elemental Analysis.
C : found 47.627 C : Calculated 47.88%
H : found 3.10% H : Calculated 3.28%
Br found 38.15% Br : Calculated 37.55%
UV Spectra :UV spectrum was recorded in methanol and is reproduced on plate no 4 (0) . λ max value are recorded 203.8 nm, 252.2 nm, 363.2 nm and 395.4 nm
corres ponding II – II* and n – II* in aurone dibromide
IR spectrum : IR spectrum was recorded in nujol and reproduced on plate No 4b
Region Frequency Co-rotation
1750-1725 1730 (s) C = o stretching in 5-membered ring
1300-1200 1280 (s) Ar-o stretching in aromatic ether
1350-1100 1180 (s) C-O stretching
1050-1010 1010(s) -OCH3 stretching in aromatic ether
750-500 750 (s) C-Br stretching
NMR spectrum
PM R spectrum who recorded in CDCL3 with TMS as an internal standard and in reproduced on plate No 4c
The observed chemical shift can be correlated as follows.
1.6 ♪ s 1H C-H
2.44 ♪ s 3H Ar-CH3
3.94 ♪ s 3H – OCH3
6.92 – 8.1♪ m 7H Ar-H
The white powder having mp=112. It gave blood red colouration with conH2So4 from analytical data, the molecular formula was found to br C17 H14 O3 and molecular mass being 266( by mass spectra.)
Elemental Analysis:
C: found 75.82% Calculated 76.13%
H: found 5.324% Calculated 5.66%
TLC studies. The RF values was found to br 0.64 – 0.67 for CCL4 as a solvent on silica gel .G plate with layer thickness of 0.3mm. The UV spectrum it is reproduced on plate No.3a. It shows λ max value 403.2, 342.8, 254.0 nm corresponding π– π* and n-π* in the compound.
IR Spectrum
IR Spectrum recorded in nujol and is reproduced on flate No.3b. The imp correlation are as follows.
Region Frequency Co-rotation
1750-1550 1700 (s) C = o stretching in cydic ketone
1700-1550 1210 (s) C=C Stretching
1310-1210 1210 (s) Ar-o strtching in aromatic rung
1280-1200 1200(s) C-O-Cstretching in 5-membered cyclic ring 1050-1010 1020 (s) -OCH3 stretching in aromatic ether
In addition to after mentioned peaks spectrum also consist peaks coversponding to other common bending, stretching vibration .
PMR spectrum
The PMR spectrum was recorded in CDCL3 in TMS a internal standard and is reproduced on plate No 36.
The observed chemical shift can be correlated as follows.
1.6 ♪ S 1H = CH
2.35 ♪ S 3H Ar – CH3
3.8 ♪ S 3H Ar – OCH3
6.87 TO 8♪ m 7H Ar – H
6.67 ♪ S 1H = CH
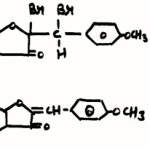 |
Scheme 1 Click here to View scheme |
Table 1
|
Sr No |
Aurone dibromide |
Aurone |
M.P.0C |
|
1 |
2 ,<Xj-dibromo-2-(4′ methoxy benzylA–5-methyl-coumaran-3-one | 2-(4′-methoxy benzylidene) -5-methyl coumaran-3-one
|
154 |
|
2 |
2, <-dibromoe-2- benzyl 5-methyl-coumaran-3-one | 2-benzylidene-5-methyl coumaran-3-one |
112 |
|
3 |
2,<-dibromo-2 (4′ methoxy-benzyl) coumaran-3-one | 2-(4’methoxy benzylidene)-4-methoxy-coumaran-3-one |
140 |
|
4 |
2 ,o-dibromo-2-(4′ methoxy
benzyl) 4-tnethoxy coumaran-3-one |
2-(4′-methoxy benzylidene-3-nitro-5-methyl coumaran-3-one |
180 |
|
5 |
2 ,o<-dibromo-2{4′ methoxy benzyl )-3-nitro-5-methyl coumaran-3-one | 2-(4′-methoxy benzylidene) -3-nitro-5-methyl coumaran -3-one |
230 |
|
6 |
2,°(-dibromo-2- benzyl -3-nitro-5-methyl coumaran-3-one | 2-(41-methoxy benzylidene)-3-bromo-5-methylcoumaran-3-one |
226 |
|
7 |
2X-di.bromo-2(4l methoxy-benzyl)-3-bromo-5-methyl coumaran-3-one | 2-(4′-methoxy benzylidene) coumaran-3-one |
176 |
|
8 |
2,o(-dibromo-2– benzyl —3-bromo-5-methyl coumaran-3-one | 2- benzylidene -3-bromo-5-methyl coumaran-3-one |
160 |
References
- T.S. Wheeley and M.P. Dodwadna-lh, Proc. Ind. Acad, Sci. India, Sect. A 2,1955, 438.
- P.A. Soni and B.J. Ghiya, Cury. Sci 41, 1972,137.
- T.K. Raja, Indian J. Chem, , Sect137 B, 1976, 812
- T.C. Sharma and M.M. Bokadia, Indiain J.Chem. Sect. B,14., 1976, 65.
- R .5. Raghavan, M. Govindrajan and K.G. Sanjeev ‘ Babu, Current Sci,48, 1979, 1072.
- D. Landini, L. Mi lest, M.L. Quadri and F. Rolla, J. Org. Chem.49, 1984, 152.
- J. Nakayama, H. Machida and’ M. Hoshino, Tetrahedron Let.24, 1983, 3001.
- Z. Goren and I , WMiner, J. Am. Chem. Soc., 165, 1983, 7764. ‘ .
- H. Alper and M, Copal, J. Org. Chem. .48, 1983, 4390.
- K, Fukunaga and M. YamagucM, Synthesis, 1981, 879.
- S,W. Sat he and B.J . Ghiya, unpub I shed work.
- V.P. Tashchi, A.F. Rukasov, T.I. Orlova, Yu. G. Putsykin and Yu. A. Baskakov, Khim, Geterotsikl. Soediri,61982, 75. (Chemabst ,S7, , 1982, 109794)
- R.T. Lalonde J, Chem. Soc. Chem. Commun 7, 1982, 401.
- V.S. Jamode, Indian J. Chem. Sect B 17, 1979,629.
- R.T. Lalonde, B.A. Horenstein, K. Schwandler, R.C. Fritz and R.A. Florence,- J. Org. Chem 48, 1983, 4049.
- A.G. Doshi and B.J. Ghiya J. Indian Chem. Soc.LX III 1986, 404-405.
- A.G. Doshi, Ph.D. thesis, Amravat i University, Amravati.

This work is licensed under a Creative Commons Attribution 4.0 International License.









