A New 2,8-Dihydroxy-1,6-Dimethoxy Xenthones from Cythula Tomesntosa
Dwarika Prasad and S.P. Sati
1Department of Chemistry Lovely Professional University. Punjab, India.
2P.G.College Gopeshwar Chamoli Uttrakhand, India.
From alcoholic extract of whole plant Cyathula tomentosa a new 2,8-dihydroxy-1,6-dimethoxyxanthone has been isolated and characterized with help of FAB-mass, 1H, 13C NMR and 2D studies & antimicrobial activities of its extract.
KEYWORDS:Cyathula tomentosa; amaranthaceae; 2,8-dihydroxy-1; 6-dimethoxyxanthone.
Download this article as:| Copy the following to cite this article: Prasad D, Sati S. P. A New 2,8-Dihydroxy-1,6-Dimethoxy Xenthones from Cythula Tomesntosa. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Prasad D, Sati S. P. A New 2,8-Dihydroxy-1,6-Dimethoxy Xenthones from Cythula Tomesntosa. Available from: http://www.orientjchem.org/?p=24681 |
Introduction
Cyathula tomentosa (Kurru) belongs to family Amaranthaceae, is a perennial under shrub occurs throughout Garhwal Himalayas up to 600-2000 meter altitude. Cyathula tomentosa have been used in snake bite and has emetic properties [1] From Cyathula capitate and Cyathula officinales isolated ecdyson content is 0.046% and 0.057% respectively [2] and Cyathula prostrata show antifungal free redical scavenging activities 2,2-diphenyl-1-picryl hydrazyl [DPPH] radical [3]. The chemical examinants at the basis of [4] has been revieweds We found no chemical analysis from literature survey on Cythula tomentosa. From the ethanolic extract of Cyathula tomentosa a new flavanone compound is isolated. The structure of compound has been through mass, 1H, 13C. NMR and 2 D-NMR spectra.
Experimental
General
1H-NMR at (400 MHz),13C-NMR at (75 MHz) TMS as internal standard, using DMSO as solvent, Columan Chromatography was carried out on silica-gel 60-120 mesh (Merck). TLC was performed on percoated silica-gel. The eluting solvent was CHCl3-MeOH spots were visualized by 7% H2SO4 followed by heating.
Plant material
The whole plant of Cyathala tomentosa were collected from Bacchear District. Chamoli Garhwal Uttrakhand in the month of October and identified by Department Botany, P.G. College Gopeshwar where Vaucher specimen was deposited.
Extraction and isolation
The air dried whole plant (3kg) was exhaustively extracted with 90% aqueous EtOH for 72 hours. The ethanolic extract was concentrated to dryness. The dry ethanolic extract was chromatographed over silica-gel using Methanol Chloroform (30:70) as eluting solvent which afforded the compound.
Result
It was crystallized from methanol as yellow amorphous solid m.p. 2360C compound was obtained as yellow amorphous solid from methanol was found to have molecular weight 288 as presumed by the presence of molecular ion peak at m/z 288 in the EI-positive mass spectrum. MS-EI+ ; m/z 288, 270, 245, 214, 199, 184, 123, 69 etc. Elemental analysis of compound found values, C=62.47%,H=2.6%, required values for C15H12O16; C=62.50%, H=4.25%; molecular weight 288 showing ten double bond equivalents (DBE) in the molecule its IR (vmax KBr): cm-1 at 3372, 2843, 16655, 1612, 1576, 1508, 1475 etc. and showed absorption at 3372, [for hydroxyl group (s)] 2843 (alkane) and 1655 and 1612 cm-1 ( for α-β unsaturated carbonyl group). The UV spectrum showed absorption maxima at 207, 230, 254, 267, 279 and 300 nm, the characteristic of xanthone[5].
The 13C-NMR spectrum of compound disclosed presence of fifteen carbon atoms out of which four were methine, two methyl and nine quaternary carbon atoms. The methine and methylene carbon atoms were promptly assigned by heteronuclear multiple quantum correlation (HMQC) experiment which allowed one bond coupling between a given proton and carbon atom.
Table 1
|
C/H |
δC |
Multiplicity |
δH (J in Hz) |
HMBC Correlation (H→C) |
|
1 |
146.4 |
C |
— |
— |
|
2 |
150.4 |
C |
— |
— |
|
3 |
125.2 |
CH |
7.59, d (9.03) |
C-1, C-2, C-4a |
|
4 |
113.9 |
CH |
7.23, d (9.03) |
C-2, C-4a, C-9a |
|
4a |
148.5 |
C |
— |
— |
|
4b |
157.6 |
C |
— |
— |
|
5 |
92.2 |
CH |
6.47, brs |
C-4b, C-6, C-7, C-8a |
|
6 |
166.7 |
C |
— |
— |
|
7 |
97.4 |
CH |
6.55, brs |
C-5, C-6, C-8, C-8a |
|
8 |
164.2 |
C |
— |
— |
|
8a |
104.5 |
C |
— |
— |
|
9 |
181.5 |
C |
— |
— |
|
9a |
115.9 |
C |
— |
— |
|
1-OMe |
61.6 |
CH3 |
4.05,s |
C-1 |
|
6-OMe |
53.9 |
CH3 |
3.74, s |
C-6 |
|
2-OH |
— |
— |
11.8, s |
C-2 |
|
8-OH |
— |
— |
13.9, s |
C-7, C-8, C-8a |
The 1H-NMR spectrum of compound disclosed the presence of two AMX- type doublets ( J=1.5 Hz) at 6.47 and 6.55 and two AB-type doublets ( J=9.0 Hz) at 7.59 and 7.23 implying the presence of two tetra-substituted benzene nuclei in the molecule. The broad singlet at 11.8 and 13.9 were attributed to two phenolic groups. Two singlets ( each for 3H) at 4.05 and 3.74 were correlated with the methoxy proton attached at benzene ring.
In the 13C –NMR spectrum of compound a downfield signal at 181.5 was attributed to alpha,beta- unsaturated carbonyl carbon, which is compatible with the carbonyl group of xanthone [6]. The carbon resonance at 146.4, 150.4, 166.7, 164.2 showed the presence of four oxygenated carbon atoms and are compatible with the 1,2,6,8-oxygenated carbon atoms of xanthone [6]. Two carbon signals at 61.1 and 53.9 in the 13C-NMR spectrum were assigned for methoxy carbon atoms.
The position of methoxy groups was authenticated to be C-1 and C-6 by comparison of 13C –NMR data with related compound [6]. The downfield chemical shift of both carbon and hydrogen of a methoxy group at δ 61.6 and 4.05 respectively as compare to the other methoxy group at 53.9 and 3.74, suggested that the former be chelated with the carbonyl group. This indicate that the methoxy group should be either at C-1 or C-8 position. The HMQC spectra of compound showed 2JCH interaction of 1H-NMR signal at 4.05 with C-1 carbon atom at 146.4 and the methoxy signal at 3.74 showed 2JCH correlation with C-6 at 166.7. These long-range correlations suggested the allocation of methoxy group at C-1 and C-6 position of molecule, The phenolic proton at 13.9 showed long range correlation with C-8, C-7 and C-8a in the HMQC spectrum confirmed the allocation of OH function at C-8 carbon atom. The other long-range correlations identified by the HMBC spectrum are showed in Table.
On the basis of the above discussed spectrum data compound was characterized as 2,8-dihydroxy-1,6-dimethoxyxanthone which was further confirmed by the comparisons of its 1H, 13C-NMR data with reported data[6].
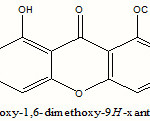 |
Scheme 1 Click here to View scheme |
References
- Gaur; R.D.” Flora of District Garhwal” Trans Media, Srinagar Garhwal 1999.
- Shu-Y, Zou-Z,Yang-A; Plant Medica, 1992,14,37.
- Cavin –A, Dyatmyko-W; Pharm.biology, 1999, 37,4, 260.
- Malikov V.M. andYuldashev M.P., Khim. Prir. Soedin, 2002, 5, 385.
- Kanamori, H., sakamoto, I., Mizuta, M., Hashimoto, K and Tanaka, O., Che. Pharm. Bull., 32, 2290 (1984).
- Elgamal, H.M.A., Soliman, S.M.H., Toth, G., halasz, J and Duddeck, H., magnetic Resonance in Chemistry, 34, 697 (1996).

This work is licensed under a Creative Commons Attribution 4.0 International License.









