Synthesis and Spectral Studies of Cobalt (II) and Nickel (II) Complexes with 18-membered Macrocyclic Ligand Derived from Malonodihydrazide
Devendra Kumar Singh* and Raman Kumar
Department of Chemistry, Rajendra College, J.P. University, Chapra - 841 301 (India).
A series of 18-membered macrocyclic complexes of metal Ni(II) of the type [M(H2Cy1-4)] have been synthesized and characterized on the basis of spectral, magnetic and conductivity studies. The macrocyclic ligands H4Cy1-4 (Fig. 1) are tetrabasic having four imido hydrogen but behave in a dibasic manner. Out of eight nitrogens, four imido and four imino, ligands utilize four imido nitrogens for square planar coordination forming four 7:6:7:6 chelate rings giving rise to a 18 membered macrocyclic framework. Nickel(II), complexes having red color are diamagnetic whereas cobalt(II) complexes with chocolate brown color are of low spin type. Low molar conductivity values in the range 7-12 ohm-1 cm2 mol-1 suggest complexes to be non-electrolytic in nature. High dilute conditions have been employed in the synthesis of macrocyclic complexes in order to dispense with polymerization reaction.
KEYWORDS:Conductivity; Malonodihydrazide complexes; Macrocyclization; Spectral study; Orientation effect
Download this article as:| Copy the following to cite this article: Singh D. K, Kumar R. Synthesis and Spectral Studies of Cobalt (II) and Nickel (II) Complexes with 18-membered Macrocyclic Ligand Derived from Malonodihydrazide. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Singh D. K, Kumar R. Synthesis and Spectral Studies of Cobalt (II) and Nickel (II) Complexes with 18-membered Macrocyclic Ligand Derived from Malonodihydrazide. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24847 |
Introduction
Worldwide attentions have been given to studies on macrocyclic complexes with tetraaza system1-4 Consequently, we in the present communication, in continuation of our earlier interest5-6, are reporting a series of cobalt(II) and nickel(II) macrocyclic complexes obtained by macrocyclization between bis-(malonodihydrazide)metal(II) precursors with diketones such as 1-phenyl propane-1,2, dione: butane-2, 3-dione: pentane-2, 3-dione: and octane 2, 3-dione. Stoichiometry of the complexes have been found to be of the type [M(H2Cy1-4)]; where M = Co(II) and Ni(II); H4Cy1 = 8, 18-dimethyl-9, 17-diphenyl- 3, 5, 12, 14-tetraoxa, 1, 2, 6, 7, 10, 11, 15, 16-octaaza cyclooctadeca (18), 7, 9, 16-tetraene, H4Cy2= 8, 9,17, 18,-tetramethyl- 3, 5, 12, 14-tetraoxa, 1, 2, 6, 7, 10, 11, 15, 16-octaaza cyclooctadeca (18), 7, 9, 16-tetraene; H4Cy3 = 8, 18-dimethyl-9, 17- diethyl-3, 5, 12, 14-tetraoxa, 1, 2, 6, 7, 10, 11, 15, 16-octaaza cyclooctadeca (18), 7,9, 16-tetraene; H4Cy4 = 8, 18-dimethyl-9, 17-dipentyl-3, 5, 12, 14-tetraoxa, 1, 2, 6, 7, 10, 11, 15, 16-octaaza cyclooctadeca1 (18), 7, 9, 16-tetraene.
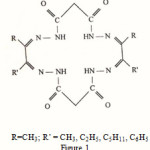 |
Figure 1: R=CH3; R’ = CH3, C2H5, C5H11, C6H5 |
Experimental
All solvents and chemicals were of reagent grade. Solvents were dried over 4Å molecular sieves and degassed with dry nitrogen before use. Synthesis and manipulation were carried out under nitrogen atmosphere.
Nickel(II) acetate tetrahydrate was prepared by dissolving the carbonate in acetic acid of BDH quality followed by crystallization. Malonodihydrazide was prepared according to literature procedure7 and recrystallized several times before use.
Although the synthetic procedure of these compounds are reported in the literature we developed a new method. Preparation of one of the complex is described here.
[Ni(H4Cy4)] 8, 18-dimethyl-9, 17-dipentyl- 3, 5, 12, 14-tetraoxa, 1, 2, 6, 7, 10, 11, 15, 16-octaaza cyclooctadeca1 (18), 7, 9, 16-tetraene.
About 2.4g (0.02 mol) of malonodihydrazide was dissolved in 200 ml of hot water. A solution of metal(II) chloride hexahydrate (2.4g, 0.01 mol) in 100 ml of water was added to malonodihydrazide solution. A greenish blue solution was formed and it was refluxed for an hour. Octane-2, 3-dione (2.84 g, 0.02 mol) dissolved in a minimum amount of hot ethanol was added to the refluxing solution in small portions. The reflux was continued for a day. A mauve coloured solid was obtained. It was cooled, filtered and washed with warm water and hot ethanol and dried in vacuo. The dry solid was analysed. Analytical data, colour, magnetic moment and conductivity data have been shown in Table – 1.
Table 1: Analytical, colour, magnetic moment and molar conductivity data for nickel(II) and cobalt(II) macrocyclic complexes of the type [M(H2Cy1-4)]
|
Compound (Colour) |
% analysis found (calculated) |
μeff
|
Ω-1cm2 mol-1
|
|||
|
M |
C |
N |
H |
|||
|
Co(H2Cy1) (Brown) |
14.63 (14.65) |
41.64 (41.69) |
27.70 (27.80) |
4.95 (4.97) |
2.2 |
8 |
|
Co(H2Cy2) (Chocolate) |
10.76 (1.79) |
52.13 (52.66) |
20.41 (20.48) |
4.37 (4.39) |
2.3 |
7 |
|
Co(H2Cy3) (Red) |
12.99 (13.09) |
42.42 (42.58) |
24.66 (24.88) |
5.31 (5.33) |
2.6 |
9 |
|
Co(H2Cy4) (Brown) |
10.25 (10.27) |
45.37 (45.92) |
19.41 (19.18) |
6.24 (6.27) |
2.1 |
11 |
|
Ni(H2Cy1) (Pink) |
14.61 (14.65) |
41.62 (41.69) |
27.66 (27.80) |
4.93 (4.97) |
Diamagnetic |
8 |
|
Ni(H2Cy2) (Violet) |
10.72 (10.79) |
52.14 (52.66) |
20.43 (20.48) |
4.34 (4.39) |
Diam |
9 |
|
Ni(H2Cy3) (Mauve) |
12.96 (13.09) |
42.44 (42.58) |
24.68 (24.88) |
5.29 (5.33) |
Diam |
10 |
|
Ni(H2Cy4) (Yellow) |
10.22 (10.27) |
45.43 (45.92) |
19.16 (19.18) |
6.19 (6.27) |
Diam |
12 |
Table 2 : Important i.r. spectral bands (cm-1) of malonodithydrazide, precursors [M(dihydrazide)2Cl2] and macrocycles [M(H2Cy1-4)]
|
Compound |
VN-H |
VS(NH2) |
VS(NH2) |
Amide I |
Amide II |
Amide III |
VC-N |
VM-X |
|
Malonodihydrazide |
3290 |
3150 |
3015 |
1690 |
1620 |
1285 |
1120 |
|
|
[Ni(dihyd)2Cl2] |
3350 |
3155 |
3010 |
1630 |
1615 |
1260 |
1100 |
460 |
|
[Ni(H2Cy1)] |
3380 |
3200 |
1650 |
1540 |
1200 |
1120 |
465 |
|
|
[Ni(H2Cy2)] |
3385 |
3205 |
1645 |
1545 |
1220 |
1110 |
470 |
|
|
[Ni(H2Cy3)] |
3375 |
3210 |
1640 |
1540 |
1215 |
1115 |
465 |
|
|
[Ni(H2Cy4)] |
3360 |
3205 |
1655 |
1550 |
1210 |
1110 |
460 |
|
|
[Co(dihyd)2Cl2] |
3320 |
3150 |
3005 |
1675 |
1620 |
1250 |
1120 |
470 |
|
[Co(H2Cy1)] |
3340 |
3180 |
1640 |
1535 |
1210 |
1110 |
455 |
|
|
[Co(H2Cy2)] |
3335 |
3185 |
1645 |
1530 |
1215 |
1105 |
450 |
|
|
[Co(H2Cy3)] |
3340 |
3180 |
1650 |
1535 |
1220 |
1125 |
460 |
|
|
[Co(H2Cy4)] |
3345 |
3190 |
1640 |
1540 |
1210 |
1110 |
465 |
Results and Dicussion
Macrocyclic complexes (Fig.2) containing a pair of α –diimine groups were synthesized by metal ion assisted template condensation of bis-(malonodihydrazide) (II) complexes with vicinal diketones like 1- phenyl propane-1, 2, dione ; butane-2, 3-dione; pentane-2, 3-dione; and octane-2, 3-dione;
The cyclisation reactions with diketones takes place readily and was marked by rapid change of colour. Besides, these reagents being comparatively less soluble in alcohol, dropwise addition of the ketones was carried out at longer intervals to avoid their precipitation and the reaction was likewise followed by observing gradual change of intensity of colour as the reaction progressed.
Infrared Spectra
Spectra have been recorded in the range 4000-400 cm-1 and important bands have been recorded in Table-2. The spectra are clearly marked by a strong band in the region 3350 to 3200 cm-1 testifying the presence of NH groups of one kind belonging to the amide functions. The lack of multiplicity of N-H stretching vibrations in this region further illustrates that the terminal NH2 groups of parent dihydrazide complexes have entered into condensation with α –diketones to yield the macrocyclic complexes.
In the fingerprint region, the macrocyclic complexes show differences in their spectral features from one another and from parent precursors. The profiles of the spectra are strikingly dependent on the substituents on the α –diimine moieties. Nevertheless the vibrational bands corresponding to amide I, amide II, amide III and C-N stretching bands are clearly displayed in the regions 1680-1630 cm-1, 1570-1515 cm-1, 1270 – 1220 cm-1 respectively.
The vibrational spectra of complexes show a well-defined band near 1680 cm-1 which posesses the attributes of stretching vibrations. A large number of bands are observed in the range 1600-1400 cm-1. The bands appear in close proximity and this is the region where we expect ring-breathing vibrations of phenyl groups. Although from intensity considerations, we have been able to identify stretching vibrations near 1600 cm-1, for the Me2Ph2O4 [16] tetraenato –(2) N4(N4)- metal (II) complexes, this band is not, however, distinct.
In the region 900-300 cm-1 we observe additional bands due to out-of-plane deformations for phenyl groups of the macrocyclic complexes besides some of the bands observed for the dihydrazide moieties. The spectra clearly demonstrate template condensation of the bis-(malonodihydrazide) complexes with the α –diketones. The structural features of these complexes involve bonding of amide groups with the metal centres and the macrocyclic ligand exists in a dianionic form. The di-anionic from of the ligands for the group of macrocycles appears to be one of the important factors for electrostatic interaction of the metal ion with the macrocyclic ligands, enclosing the metal ion in the macrocyclic cavity forming 6-membered chelate rings with the amide moieties and 7-membered rings involving α –diimine groups. A strong band in the region 460-470 cm-1 in the spectra of all the macrocyclic complexes has been assigned to M-N stretching vibration; on8.
Electronic spectra and magnetic properties
The nickel(II) macrocyclic complexes [Ni(H2Cy1-4)] are diamagnetic and their electronic spectra show two intense bands one in the region 19000 to 21000 and another near 27000 cm-1. The transitions are assigned to 1A2g 1A1g and 1B1g 1A1g under a square planar environment having NiN4 chromophore. The higher energy band is, however, more intense and the intensity is believed to arise due to metal ligand π* charge transfer transitions9.
The cobalt (II) complexes are of low spin type and are found to possess magnetic moments in the range 2.2 to 2.7 B.M. Electronic spectra of the complexes are similar and most of them exhibit two transition near 20,000 and 25000 cm-1 respectively. The lower energy band has been assigned to the transition 2A1g ground term to one of the higher energy term , 2A2g or 2Eg , the split components of the orbitally triplets 2T1g term. The higher energy band is more intense and indicates its origin due to metal ligand (π*) charge transfer transition10,11.
Molar conductivity measured in DMF solution lies in the range 7-12 Ω-1 cm2 mol-1 and indicate the macrocyclic complexes to be non-electrolytic in nature.
Table 3: Electronic spectral bands (in cm-1) of the macrocyclic nickel(II) and cobalt(II) complexes of the type [M(H2Cy1-4)]
|
S.N. |
Complexes |
L.F. Bands |
C.T. Band |
μeff (in B.M.) |
|
01 |
Ni(H2Cy1) |
20,000 25,000 |
28,000 |
Diamagnetic |
|
02 |
Ni(H2Cy2) |
20,800 25,200 |
28,000 |
Diamagnetic |
|
03 |
Ni(H2Cy3) |
19,300 22,500 |
27,000 |
Diamagnetic |
|
04 |
Ni(H2Cy4) |
20,500 22,700 |
27,500 |
Diamagnetic |
|
05 |
Co(H2Cy1) |
19,000 |
25,000 |
2.19 |
|
16 |
Co(H2Cy2) |
19,500 |
24,800 |
2.37 |
|
07 |
Co(H2Cy3) |
19,600 |
25,600 |
2.65 |
|
08 |
Co(H2Cy4) |
19,400 |
25,300 |
2.48 |
On the basis of information received from spectral, magnetic and conductivity studies, macrocycles have been assigned structure as shown in Fig. 2.
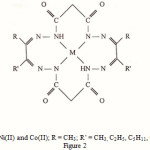 |
Figure 2: M = Ni(II) and Co(II); R = CH3; R’ = CH3, C2H5, C5H11, C6H5 |
References
- S. Chandra, L.K. Gupta and Sangeetika, Synth. React. Inorg. Metal. Org. Chem., 34, 1591 (2004).
- S. Chandra and L.K. Gupta, Spectrochim. Acta, 60A, 1563 (2004).
- S. Chandra and A. Gautam, J. Ind. Chem. Soc., 85, 980 (2008).
- R.R. Jha and N.Sahu, Asian J. Chem., 20, 6301 (2008).
- H.C. Rai and B.K. Rai, Asian J.Phys. 12, 133 (2003).
- H.C. Rai, J.Tiwary and Rekha Kumari, Ind. J. Chem 27A, 1054 (1988).
- B.S. Furniss et al., Vogel’s Textbook of Practical Organic Chemistry, ELBS/Longman, England, 1125 (1978).
- S.Surendra Babu, P.G. Krishna, K. Hussain Reddy and G.H. Philip, Indian J. Chem., 47A, 1663 (2008)
- A.B.P. Lever, Inorganic Electronic Spectroscopy, Elsevier Pub. Co., Amsterdam, 343 (1968)
- M. Nicolini, C. Pecile and A. Turco, Coord. Chem. Rev., 1, 133 (1966)
- A.B.P. Lever, J. Lewis and R.S. Nyholm, J. Chem. Soc., 2552 (1963).

This work is licensed under a Creative Commons Attribution 4.0 International License.









