Synthesis and Properties of Biopolymer based on Carboxymethyl Cellulose-g-Poly(N-vinyl pyrollidin-co-2-Acrylamido-2-methyl propan Sulfonic Acid as Superabsorbent hydrogels
Mohammad Sadeghi1* and Mojgan Yarahmadi2
¹Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak (Iran) ²Department of English, Humaniti Faculty, Islamic Azad University, Arak Branch, Arak (Iran).
Article Received on :
Article Accepted on :
Article Published : 05 Mar 2011
Novel biopolymer-based superabsorbent hydrogels were prepared by grafting crosslinked poly(NVP-co-AMPS) chains onto CMC backbones through a free radical polymerization method. A proposed mechanism for superabsorbent hydrogel formation was suggested and the hydrogel structure was confirmed using FTIR spectroscopy. The morphology of the samples was examined by scanning electron microscopy (SEM). The effect of concentration of MBA as well as NVP/AMPS weight ratio on swelling capacity of hydrogel was also studied. Furthermore, the water absorbency of hydrogels was measured in solutions with pH ranged 1 to 13. The CMC-based hydrogel exhibited a pH-responsiveness character so that a swelling-deswelling pulsatile behavior was recorded at pHs 2 and 8. Additionally, the hydrogels exhibited salt-sensitivity and cation exchange properties.
KEYWORDS:CMC; hydrogels; swelling; N-vinyl pyrollidin; 2-Acrylamido-2-methyl propan Sulfonic acid
Download this article as:| Copy the following to cite this article: Sadeghi M, Yarahmadi M. Synthesis and Properties of Biopolymer based on Carboxymethyl Cellulose-g-Poly(N-vinyl pyrollidin-co-2-Acrylamido-2-methyl propan Sulfonic Acid as Superabsorbent hydrogels. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Sadeghi M, Yarahmadi M. Synthesis and Properties of Biopolymer based on Carboxymethyl Cellulose-g-Poly(N-vinyl pyrollidin-co-2-Acrylamido-2-methyl propan Sulfonic Acid as Superabsorbent hydrogels. Available from: http://www.orientjchem.org/?p=11633 |
Introduction
Superabsorbent hydrogels are hydrophilic, three dimensional networks. They exhibit the ability to highly swell in water, saline or biological fluids and retain a significant fraction of them within their structure, but they do not dissolve in water (1). Superabsorbent hydrogels have great advantages over traditional water-absorbing materials such as cotton, pulp, and sponge. They are widely used in sanitary goods like disposable diapers and hygienic napkins. They are also used in soil conditioning and improving water retention capability of soil in agriculture and horticulture (2).
Research on superabsorbents was initiated by the development of a starch-based superabsorbent by the U.S. Department of Agriculture, NorthernRegionalResearchCenter, in the late 1960s (3). Since then, modification of natural materials such as starch (4), cellulose (5, 6), and protein (7) have been utilized to prepare superabsorbents.
Hydrogels responding to external stimuli such as heat, pH, electric field, chemical environments, etc, are often referred to as “intelligent” or “smart” hydrogels. These hydrogels have important applications in the field of medicine, pharmacy, and biotechnology (8-10). Following a continuous research on modification of carboxymethylcellulose sodium salt (CMC) (11-14), in this paper, we report synthesis and characterization of a smart superabsorbing hydrogel from CMC-g-(PNVP-co-PAMPS). Effect of the grafting and the subsequent reaction variables on the swelling properties as well as the salt- and pH-sensitivity of the hydrogels were investigated in detail.
Experimental
Materials
The polysaccharide used in this work was carboxymethylcellulose (CMC) with D.S. 0.52. Ceric ammonium nitrate (CAN) was purchased from Merck and was used without purification. It was as freshly prepared 0.1 M solution in 1N HNO3. N-vinyl pyrollidin,(NVP, Merck) and 2-Acrylamido-2-methyl propan Sulfonic acid(Fluka, Buchs, Switzerland) were used after distillation for removing inhibitor. All other chemicals were of analytical grade.
Preparation of Hydrogel
A pre-weighed amount of carboxymethylcelloluse(1.0 g) was dissolved in 50 mL distilled water. Then the solution was added to a 1-L reactor equipped with a mechanical stirrer (RZR 2021, a three-blade propeller type, Heidolph, Schwabach, Germany) and stirred (300 rpm) for 10 min. The reactor was placed in a thermostated water bath to control the reaction temperature at 65oC. Then, N-vinyl pyrollidin, (1.0-4.0 g) and 2-Acrylamido-2-methyl propan Sulfonic acid, (4.0-1.0 g) , ceric ammonium nitrate (0.1 g, dissolved in 5 mL water-HNO3) and methylene bisacrylamide (0.05-0.20 g, dissolved in 5 mL water) were added simultaneously to the reactor. The temperature was maintained at 65oC and the reaction mixture was stirred continuously (300 rpm) for 90min. At the end of the propagation reaction, the gel product was poured into ethanol (300 mL) and was dewatered for 24 h. Then, the product was cut into small pieces, washed with 500 mL ethanol and filtered. The particles were dried in an oven at 50oC for 12 h. After grinding, the powdered superabsorbent composite was stored in absence of moisture, heat and light.
Swelling and Deswelling Measurements
An accurately weighed sample (0.20 g) of the powdered superabsorbent with average particle sizes between 40-60 mesh (250–350 μm) was immersed in distilled water (200 mL) or desired salt solution (100 mL) and allowed to soak for 3 h at room temperature. The equilibrium swelling (ES) capacity was measured twice at room temperature according to a conventional tea bag (i.e. a 100 mesh nylon screen) method14 and using the following formula:
![]()
The deswelling water ratio of each sample was evaluated from the following equation:
![]()
where Wt0 and Wt are the initial weight of the fully swollen sample and the weight of sample at the deswelling time, t, respectively.
Absorbency at Various pHs
Individual solutions with acidic and basic pHs were prepared by dilution of NaOH (pH 10.0) and HCl (pH 1.0) solutions to achieve pH≥6.0 and pH<6.0, respectively. The pH values were precisely checked by a pH-meter (Metrohm/620, accuracy ±0.1). Then, 0.5 g (± 0.001 g) of the dried hydrogel was used for the swelling measurements according to Eq. 1. To study the pH-reversibility of the hydrogels, solutions with pH 2.0 and 8.0 were used. Swelling capacity of the hydrogels at each pH was measured according to Eq. 1 at consecutive time intervals (30 min).
Results and Discussion
Synthesis and Spectral Characterization
Scheme 1 shows a simple structural proposal of the graft copolymerization of N-vinyl pyrollidin and 2-Acrylamido-2-methyl propan Sulfonic acid monomers on the CMC backbones and crosslinking of the graft copolymer. In the first step, the thermally dissociating initiator, i.e. CAN, is decomposed under heating (65oC) and Then pair redox ce3+ -ce4+ disconnect C-C bond from the CMC backbones to form corresponding macroinitiators. These macroradicals initiate grafting of NVP and AMPS onto CMC backbones leading to a graft copolymer. Crosslinking reaction also occurred in the presence of the crosslinker, i.e. MBA.
FTIR spectroscopy was used for identification of the hydrogel. Figure 1 shows the IR spectra of the CMC and the resulted hydrogel. The band observed at 1623 cm-1 and can 1430 cm-1 be attributed to C=O stretching and bending in carboxylate(COO –) functional groups of substrate backbone (Figure 1a). The broad band at 2500-3500 cm-1 is due to stretching of –OH groups of the CMC. In the spectra of the hydrogel the characteristic bands at 1553 cm-1 , 1230cm-1 and 1670 cm-1 were attributed to N-H , S=O (AMPS monomer) and C=O (vinyl pyrollidin) stretching respectively.
To obtain additional evidence of grafting, a similar polymerization was conducted in the absence of the crosslinker. After extracting the homopolymers, PNVP or PAMPS and unreacted monomers using a cellophane membrane dialysis bag (D9402, Sigma–Aldrich), an appreciable amount of grafted CMC (87%) was observed. The graft copolymer spectrum was very similar to Figure 1b. Also according to preliminary measurements, the sol (soluble) content of the hydrogel networks was as little as 1.6 %. This fact practically proves that all NVP and AMPS are involved in the polymer network. So, the monomers percent in the network will be very similar to that of the initial feed of reaction.
One of the most important properties that must be considered is hydrogel microstructure morphologies. Figure 2 shows the scanning electron microscope images of the hydrogel. This picture verifies that the synthesized polymer in this work have a porous structure. It is supposed that these pores are the regions of water permeation and interaction sites of external stimuli with the hydrophilic groups of the graft copolymers.
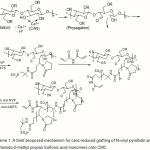 |
Scheme 1: A brief proposed mechanism for ceric-induced grafting of N-vinyl pyrollidin and 2-Acrylamido-2-methyl propan Sulfonic acid monomers onto CMC. |
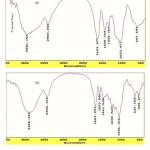 |
Figure 1: FTIR spectra of CMC (a) and crosslinked CMC-g-(PNVP-co-PAMPS) hydrogel (b). |
 |
Figure 2: SEM photograph of the hydrogel. Surfaces were taken at a magnification of 2500, and the scale bar is 10 μm. |
Effect of MBA Concentration
The effect of crosslinker concentration (Cc) on swelling capacity of crosslinked CMC-g-(PNVP-co-PAMPS) was investigated. As shown in Figure 3, more values of absorbency are obtained by lower Cc. Such a well-known behavior reported by pioneering scientists(9). In fact, higher Cc decrease the free space between the copolymer chains and consequently the resulted highly crosslinked rigid structure can not be expanded and hold a large quantity of water(10). This power law behavior between swelling capacity and MBA concentration (Eq. 3) was conducted from Figure 3.
Swelling capacity ≈ k[MBA]–n (3)
The k and n in Eq. 3 are constant values for an individual superabsorbent. The n value represents the extent of the sensitivity of the hydrogel to the crosslinker content, while the k value gives an amount useful for comparing the extent of swelling versus fixed crosslinker content. The k=1.33 and n=1.09 is obtained from the curve fitted with Eq. 3.
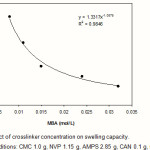 |
Figure 3:Effect of crosslinker concentration on swelling capacity. Reaction conditions: CMC 1.0 g, NVP 1.15 g, AMPS 2.85 g, CAN 0.1 g, 65 °C, 90 min. |
Effect of AMPS/ NVP Weight Ratio
The swelling capacity of superabsorbent as a function of the co-monomers ratio is illustrated in Figure 4. It is known that a high concentration of charged ionic groups existing in copolymer chains along with increase of AMPS in the hydrogel increases the swelling capacity due to osmosis and charge repulsion(10). In other words, the presence of more ionic groups in the polymer chains results in increased swelling, because the ionic groups are more strongly solvated than non-ionic groups in the aqueous medium. Similar conclusions were reported by other investigators(11,12).
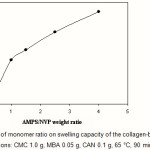 |
Figure 4: Effect of monomer ratio on swelling capacity of the collagen-based hydrogels.
Reaction conditions: CMC 1.0 g, MBA 0.05 g, CAN 0.1 g, 65 °C, 90 min. |
pH-sensitivity and Pulsatile Behavior
Equilibrium swelling studies indicated that the ionic hydrogels were sensitive to environmental pH. Therefore, in this series of experiments, swelling ratio for the synthesized hydrogels was measured in different pH solutions ranged from 1.0 to 13.0 (Figure 5). Since the swelling capacity of all “anionic” hydrogels is appreciably decreased by addition of counter ions (cations) to the swelling medium, no buffer solutions were used(13). Therefore, stock NaOH (pH 10.0) and HCl (1.0) solutions were diluted with distilled water to reach desired basic and acidic pHs, respectively. Maximum swelling (87 g/g) was obtained at pH 8. In acidic media, the most of solfonic groups are protonated, so decreased repulsion of anionic groups leads to a decreased swelling ratio. At higher pHs (5-8), some of solfonic groups are ionized and the electrostatic repulsion between SO3– groups causes an enhancement of the swelling capacity. The reason of the swelling-loss for the highly basic solutions is “charge screening effect” of excess Na+ in the swelling media which shield the solfonic anions and prevent effective anion-anion repulsion(15).
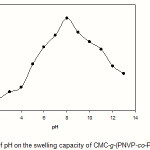 |
Figure 5: Effect of pH on the swelling capacity of CMC-g-(PNVP-co-PAMPS) hydrogel. |
The CMC-g-(PNVP-co-PAMPS) hydrogels were also showed reproducible swelling-deswelling cycles at pH 2.0 and 8.0 as demonstrated in Figure 6. At pH 8.0, the hydrogel swells up to 76 g/g due to anion-anion repulsive electrostatic forces, while at pH 2.0, it shrinks within a few minutes due to protonation of solfonic groups(16). This sharp swelling-deswelling behavior of the hydrogels makes them as suitable candidate for controlled drug delivery systems. Such on-off switching behavior as reversible swelling and deswelling has been reported for other ionic hydrogels(17,18).
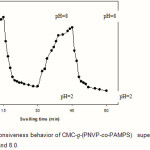 |
Figure 6: The pH-responsiveness behavior of CMC-g-(PNVP-co-PAMPS) superabsorbing hydrogel in solutions with pH 2.0 and 8.0. |
Swelling in Various Salt Solutions
Swelling capacity in salt solutions is of prime significance in many practical applications such as personal hygiene products and water release systems in agriculture. The swelling ability of “anionic” hydrogels in various salt solutions is appreciably decreased compared to the swelling values in distilled water. This well-known undesired swelling loss is often attributed to a “charge screening effect” of the additional cations which causing a non-perfect anion–anion electrostatic repulsion.15 Also, in salt solution the osmotic pressure resulting from the difference in the mobile ion concentration between gel and the aqueous phases is decreased and consequently the absorbency amounts are diminished(19). In the present study, swelling capacity was studied in various chloride salt solutions (Figure 7). As shown in the Figure 8, multivalent cations decrease the swelling capacity considerably. This dramatic decrease of water absorbency in multivalent cationic solutions could be related to the complexing ability of the solfonic groups inducing the formation of intramolecular and intermolecular complexes, which resulted in an increase in the crosslinking density of the network(21).
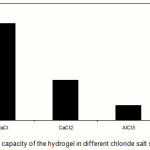 |
Figure 7: Swelling capacity of the hydrogel in different chloride salt solutions (0.15 M). |
Since the collagen-based hydrogels are comprised solfonic groups, they exhibit various swelling capacity in different salt solutions with same concentrations. These swelling changes are due to valency difference of salts. The networks contain PAMPS chains with solfonic groups that can interact with cations. As given in Figure 7, the swelling capacity of the hydrogels in CaCl2 solution is lower than that of in NaCl solutions(23). As mentioned above, in the presence of the bivalent calcium ions, the crosslinking density increases because of a double interaction of CaCl2 with carboxylate groups leading to ionic crosslinking. The swelling–deswelling cycle of the hydrogel in sodium and calcium salts are shown in Figure 8. In sodium solution, swelling of the hydrogel is increased with time. When this hydrogel is immersed in calcium chloride solution, it deswells to a collapsed form(21,22)). When the shrinked hydrogel is immersed in sodium chloride solution again, the calcium ions are replaced by sodium ions.
This ion exchange disrupts the ionic crosslinks leading to swelling enhancement. As a result, when hydrogel is treated alternatively with NaCl and CaCl2 solutions with equal molarity, the swelling reversibility of hydrogel is observed. This chemical behavior of hydrogel is resulted from the ion exchange ability of the solfonic groups(24,26).
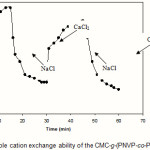 |
Figure 8: Reversible cation exchange ability of the CMC-g-(PNVP-co-PAMPS)hydrogel. |
Conclusion
In the present study, CMC-g-(PNVP-co-PAMPS) superabsorbent hydrogel was synthesized in an aqueous solution using a ceric ammonium nitrate initiator and a hydrophilic crosslinker. Swelling capacity of the hydrogels is affected by the crosslinker (MBA) concentration and monomer ratio, so that the swelling is decreased by increasing the MBA concentration and increased with increasing the AMPS/NVP ratio. Swelling capacity of CMC-g-(PNVP-co-PAMPS) hydrogel in various pH solutions (1-13) as well as swelling-deswelling behavior of the product exhibited high pH-sensitivity and reversible pH-responsiveness properties. This superabsorbent network intelligently responding to pH may be considered as an excellent candidate to design novel drug delivery systems. Swelling-loss in salt solutions, in comparison with distilled water, can be attributed to charge screening effect and ionic crosslinking for mono- and multi-valent cations, respectively. The swelling capacity in CaCl2 is much lower than that in NaCl solution and distillated water.
References
- Buchholz, F. L.; Graham, A. T. Modern Superabsorbent Polymer Technology; Elsevier: Amsterdam, (1997).
- Peppas, L. B.; Harland, R. S. In Absorbent Polymer Technology; Elsevier: Amsterdam,( 1990).
- Po, R. J Macromol Sci Rev Macromol Chem Phys, C34, 607, (1994).
- Hoffman, A. S. In Polymeric Materials Encyclopedia; Salamone, J. C., Ed.; CRC Press: Boca Raton, FL,; Vol. 5, p 3282 (1996).
- Yazdani-Pedram, M.; Retuert, J.; Quijada, R. Macromol Chem Phys, 201, 923(2000).
- Sugahara, Y.; Takahisa, O. J Appl Polym Sci, 82, 1437(2001).
- Patel, G. M.; Trivedi, H. C. Eur Polym J, 35, 201 (1999).
- Silong, S.; Rahman, L. J Appl Polym Sci, 76, 516(2000).
- Kost, J. In Encyclopedia of Controlled Drug Delivery; Mathiowitz, E., Ed.; Wiley: New York, Vol. 1, p 445(1999).
- Peppas, N. A.; Mikes, A. G. In Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, Vol. 1, (1986)
- Fanta, G. F.; Burr, R. C.; Doane, M. W. ACS Symp Ser, 187, 195(1982).
- Yamaguchi, M.; Watamoto, H.; Sakamoto, M. Carbohydr Polym, 9, 15(1988).
- Rodehed, C.; Ranby, B. J Appl Polym Sci, 32, 3323(1986).
- Lim, D. W.; Whang, H. S.; Yoon, K. J. J Appl Polym Sci, 79, 1423(2001).
- Silverstein, R. M.; Webster, F. X. Spectrometric Identification of Organic Compounds, 6th ed.; Wiley: New York, (1998).
- Castel, D.; Ricard, A.; Audebert, R. J Appl Polym Sci, 39, 11(1990).
- Lim, D. W.; Whang, H. S.; Yoon, K. J. J Appl Polym Sci, 79, 1423(2001).
- Lee, W. F.; Yuan, W. Y. J Appl Polym Sci, 77, 1760(2000).
- Park, S. E.; Nho, Y. C.; Lim, Y. M.; Kim, H. J Appl Polym Sci, 91, 636(2004).
- Burugapalli, K.; Bhatia, D.; Koul, V.; Choudhary,V. J Appl Polym Sci, 82, 217(2001).
- Lu, S.; Duan, M.; Lin, S. J Appl Polym Sci, 79, 1665(2001).
- Pourjavadi, A.; Sadeghi, M.; Hosseinzadeh, H. Polym Adv Technol, 15, 645(2004).
- Richter, A.; Bund, A.; Keller, M.; Arndt, K. Sens Actuators B, 99, 579(2004).
- Kiatkamjorwong, S.; Phunchareon P. J Appl Polym Sci, 72, 1349(1999).
- Wen-Fu, L.; You-Min, T. J Appl Polym Sci, 72, 1221(1999).
- Omidian, H.; Hashemi, S. A.; Sammes, P. G.; Meldrum, I. Polymer, 40, 1753(1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.









