Synthesis and Hydrolysis Kinetic Study of Few Co-drugs of Propranolol and other Antihypertensive Drugs
Mayukh Baidya* and Amit Kumar Das
Departmental of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, Bangalore - 560 035 (India).
The different acyl halide analogs of propranolol were synthesized by reacting Propanolol with different acyl anhydrides in toluene medium. The derivatives were reacted with thionyl chloride to get propranolol hemi acyl chloride. Finally the co-drugs were synthesized by reacting propranolol hemi acyl chloride with different classes of antihypertensive drugs like Nifedepine (PSN,PMN,PPN), Hydrochlorthiazide (PSH, PMH, PPH) and Acetazolamide(PSA, PMA, PPA) by ester linkage. The structure of the synthesized derivative of propranolol analogs were confirmed by MP, TLC, IR and NMR data.
KEYWORDS:Propranolol; Acyl anhydrided; Antihypertensive drugs
Download this article as:| Copy the following to cite this article: Baidya M, Das A. K. Synthesis and Hydrolysis Kinetic Study of Few Co-drugs of Propranolol and other Antihypertensive Drugs. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Baidya M, Das A. K. Synthesis and Hydrolysis Kinetic Study of Few Co-drugs of Propranolol and other Antihypertensive Drugs. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24796 |
Introduction
A co-drug is comprised two or more covalently bound moieties, wherein each moiety is a residue of a constituent compound having a biological activity, or a prodrug form thereof, wherein said codrug is cleaved under physiological conditions to regenerate each constituent compound1. In present investigation the various co-drugs were made by reacting propranolol with other class of antihypertensive drugs like nifedepine, hydrochlorthiazide and acetazolamide through anhydride (Succinic, Maleic, Phthalic) bridge. The reactions were monitored by single spot TLC and the structures of the compounds were confirmed by MP, TLC, IR and NMR data (Table II and III)
Experimental
Melting points were recorded in open capillary tubes and are uncorrected. The IR spectra were carried out using KBr pellets at IISc, Bangalore. The NMR spectra of the compounds were carried out in Bruker spectrospin-200 NMR spectrophotometer at IISc, Bangalore.(Table II and III)
Synthesis of Propranolol hemi ester
Mixture of 1 eq of propranolol hydrochloride and 1 eq.of acyl anhydride (Succinic, Maleic, Phthalic) were refluxed in redistilled toluene for 7 hrs with occasional strring. The newly-formed products were separated as oil during the reaction. The toluene was decanted and the product was crystallized from acetone. The product showed the single spot on TLC using methanol and n-butanol (1:1) as mobile phase.2
Synthesis of propranolol hemi acyl chloride
0.5g of dry propranolol hemiester was taken into a flask fitted with reflux condenser. To this 5 ml of redistilled thionyl chloride was added carefully. The reaction mixture was refluxed for 30 min. Cotton wool was placed in top of the condenser to reduce the entry of moisture. The excess thionyl chloride was distilled at 780C from the reaction mixture. The liquid was cooled and collected the solid.2
Synthesis of codrug of propranolol
Co-drug of propranolol with nifedepine through amidation reaction
Equimolar quantity of propranolol hemi acyl chloride and nifedepine were taken in round bottom flask. 15ml Toluene and 1 ml Triethyl amine were added to the reaction mixture. The reaction mixture was refluxed for 30 min and cooled. The dark color solution was filtered and concentrated. 3
Co-drug of propranolol with hydrochlorthiazide and acetazolamide through amidation reaction
1.7mM of each hydrochlorthiazide and acetazolamide was dissolved in 10 ml of distilled water in a separate RBF. The pH of the solution was adjusted to 7 after that 8 ml of Dioxan were added slowly to the solution. The solution was kept at 8 degree C during this entire procedure
The solution containing the propranolol hemi acyl chloride was added drop wise to hydrochlorthiazide and acetazolamide solution with constant stirring. Maintained the temperature at 8 degree C throughout the reaction. The pH was maintained between 8.5 to 9.5 in both the flask by addition of a few drops of sodium hydroxide (1N). The pH of the solutions was checked frequently and maintained. The reaction was considered to be completed when the pH did not drop on the addition of the anhydride solution in both the reaction vessels. The reaction mixture was then allowed to stand at 8 degree C for an additional 20-30 min. The precipitate was obtained, collected through filtration.
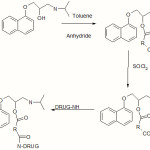 |
Scheme 1 Click here to View Scheme |
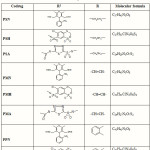 |
Table 1: Structure of synthesized codrug |
The completion of reactions were confirmed by taking TLC using ethyl acetate : n-hexane (9:1) mobile phase and melting point was checked.4
Results and Dissection
Hydrolysis at pH 7.4
Twelve co-drugs ((PSN, PSH, PSA, PMN, PMH, PMA, PPN, PPH, and PPA) were synthesized out of which three derivatives, i.e., PSH, PMH, PPH have shown hydrolysis half life 30, 32 and 34 min for hydrochlorthiazide and 5.4, 5.3 and 2.2 hr for propranolol hemiester at 370C.
The compound PSA, PMA and PPA has shown hydrolysis half life 38, 32 and min for acetazolamide and 5.4, 5.2 and 3.0 hr for propranolol hemi ester at 370C.
Similarly the compound PSN, PMN and PPN has shown hydrolysis half life 33, 36 and 30 min for nifedepine and 5.1, 5.3 and 5.2 hr for propranolol hemi ester at 370C.
All the derivatives mentioned above has shown the hydrolysis at pH-7.4 and the rate of chemical hydrolysis were significant. Table IV, V and VI 5,6
Table 2: Analytical data of synthesized compound
|
Codrug |
MP 0C |
Rf value of TLC |
% yield |
|
PSN |
265 |
0.69 |
50 |
|
PSH |
286 |
0.79 |
65 |
|
PSA |
280 |
0.61 |
65 |
|
PMN |
268 |
0.52 |
46 |
|
PMH |
282 |
0.49 |
56 |
|
PMA |
275 |
0.65 |
70 |
|
PPN |
260 |
0.52 |
50 |
|
PPH |
273 |
0.45 |
75 |
|
PPA |
258 |
0.59 |
55 |
Table 3: NMR and IR Data Of Synthesized codrug
|
Codrug |
NMR Data |
IR Data |
PSN |
d7.7(d,2H ArH), d7.67(d,2H ArH), d7.52(d,2H ArH), d7.45(t,1H ArH), d7.3(t,2H ArH), d7.25(t,2H ArH), d5.6(1H NH), d3.5(s,6H OCH3), d3.1(m,2H AlH), d2.8(s,4H AlH), d2.51(s,6H AlH), d2.3(6H AlH), d1.4(t,4H AlH), d1.25(d,4H AlH). | 3331.04 cm-1(20 NH str ), 3099.68.01cm-1 (C-H aromatic), 2953.19cm-1 (C-H aliphatic str) ,1683.81 cm-1(Amide C=O str),1646.66(ester C=O) 1529.56 cm-1(C=C str aromatic), 1101.27 cm-1(-O- str). |
| PSH | 1.2d (CH3,d,6H), 2.5d (CH2, d, 4H), 3.4d( CH2, t, 2H), 6d(CONH, s, 1H), 7-8.5d( aromatic H, m, 11H)8.6d( ring NH, s, 1H), 8.9d( ring NH, s,1H), | 3275.88 cm-1(20 NH str ), 3361 cm–1(ring NH str) 2926cm-1, (C-H aliphatic str) 3223cm-1 (C-H aromatic),1679 cm-1 (C=O str), 1456.90 cm-1(C=C str aromatic), 1367.82 cm-1(SO2 str). |
| PSA | 1.3d (CH3,d,6H), 3.2d (CH2, d, 4H), 6.3d(CONH, s, 1H), 8.6d( ring NH, s, 1H), 8.9d( ring NH, s,1H), 3.5d( CH2, d 2H), 7-8.5d (aromatic H,m,7H). | 3289.68cm-1(20 NH str ), 2926cm-1 (C-H aliphatic str) 3223.3cm-1 (C-H aromatic),1679cm-1(C=O str),1456.90 cm-1(C=C str aromatic), 1367.82 cm-1(SO2 str). |
| PMN | d7.68-7.66(m,5H ArH), d7.51-7.26(m,6H ArH), d5.71(1H NH), d3.83(6H CH2), d3.58(6H OCH3), d3.11(7H AlH), d2.67(6H AlH), d2.34(5H AlH). | 3330.80 cm-1(20 NH str ), 3100.87cm-1 (C-H aromatic), 2953.20cm-1 (C-H aliphatic str) ,1683.67 cm-1(Amide C=O str),1647.07(ester C=O) 1529.16 cm-1(C=C str aromatic), 1101.73 cm-1(-O- str). |
| PMH | 1.3d (CH3,d,6H), 2.4d (CH2, d, 4H), 4d( CH, d, 2H), 6.2d(CONH, s, 1H), 7.6d( ring NH, s, 1H), 7.7d( ring NH, s,1H),7.8-8.5d( aromatic H, m, 11H) | 3329 cm-1(20 NH str ), 2953.03cm-1 (C-H aliphatic str) ,1683.38 cm-1(Amide C=O str),1647.58(ester C=O) 1529.23 cm-1(C=C str aromatic), 1104.784cm-1(-O- str). |
| PMA | 1.3d (CH3,d,6H), 3.2d (CH2, d, 4H), 6.3d(CONH, s, 1H), 8.6d( ring NH, s, 1H), 8.9d( ring NH, s,1H), 3.5d( CH2, d 2H), 7-8.5d (aromatic H,m,7H). | 3289.67cm-1(20 NH str ), 2965.27cm-1 (C-H aliphatic str) 3221.35cm-1 (C-H aromatic),1679.25cm-1(C=O str),1455.95 cm-1(C=C str aromatic), 1367.85 cm-1(SO2 str). |
| PPN | d7.69(d,2H ArH), d7.67(d,2H ArH), d7.52(d,2H ArH), d7.50(d,2H ArH), d7.47-7.45(7H ArH), d5.72(1H NH), d3.59(s,6H AlH), d3.13-3.08(m,1H AlH), d2.69-2.65(4H AlH), d2.53(12H AlH), d1.42-1.38(5H AlH), d1.25(1H AlH). | 3331.42 cm-1(20 NH str ), 3078.01cm-1 (C-H aromatic), 2953.35cm-1 (C-H aliphatic str) ,1680.44 cm-1(Amide C=O str),1647.38(ester C=O) 1529.64 cm-1(C=C str aromatic), 1101.11 cm-1(-O- str). |
| PPH | 1.3d (CH3,d,6H), 3.2d (CH2, d, 4H), 6.3d(CONH, s, 1H), 8.6d( ring NH, s, 1H), 8.9d( ring NH, s,1H), 3.5d( CH2, d 2H), 7-8.5d(aromatic H, m,11H) | 3301.9 cm-1(20 NH str ), 2785cm-1 (C-H aliphatic str). 3082.3cm-1 (C-H aromatic),1686 cm-1(C=Ostr),1420.40 cm– 1(C=C str aromatic), 1365.8 cm-1(SO2 str). |
| PPA | 1.3d (CH3,d,6H), 3.5d (CH2, t, 2H), 6.3d(CONH, s, 1H), 8.9d( ring NH, s,1H), 4.2d( CH2, t, 2H), 8-9.5d( aromatic H, m, 7H). | 3277.1cm-1(20 NH str ), 2926cm-1 (C-H aliphatic) 3104.1cm-1 (C-H aromatic),1763cm-1(C=O str),1458 cm-1(C=C str aromatic),1336 cm-1(SO2 str). |
Hydrolysis at pH 1.2
None of the synthesized codrugs were hydrolyzed at pH 1.2 at 370C (Table VI, V and VI.)
Table 4: Hydrolysis half life of codrugs of Propranolol and Hydrochlorthiazide
|
codrug |
pH 1.2 |
pH 7.4 |
|
|
Propranolol hemi ester |
Hydrochlorthiazide |
||
|
PSH |
No hydrolysis |
5.2 hr |
30 min |
|
PMH |
No hydrolysis |
5.3 hr |
32 min |
|
PPH |
No hydrolysis |
2.2 hr |
34 min |
The above hydrolytic study indicates that none of the derivatives is hydrolyzed at gastric pH
Table 5: Hydrolysis half life of codrugs of Propranolol and Acetazolamide
|
codrug |
pH 1.2 |
pH 7.4 |
|
|
Propranolol hemi ester |
acetazolamide |
||
|
PSA |
No hydrolysis |
5.4 hr |
38 min |
|
PMA |
No hydrolysis |
5.2 hr |
32 min |
|
PPA |
No hydrolysis |
3.0 hr |
40 min |
Table 6: Hydrolysis half life of codrugs of Propranolol and Nifedepine
|
Codrug |
pH 1.2 |
pH 7.4 |
|
|
Propranolol hemi ester |
Nifedepine |
||
|
PSN |
No hydrolysis |
5.1 |
33 |
|
PMN |
No hydrolysis |
5.3 |
36 |
|
PPN |
No hydrolysis |
5.2 |
30 |
Conclusion
Since the synthesized analogs have been made by combining with two different antihypertensive drugs with ester linkage by different anhydride, they may act as new class of co-drug which are metabolized slowly by liver enzyme (first pass) or metabolized in the blood circulation and activated inside the body and may enhance the duration of action also. The hydrolysis kinetics of the synthesized product has shown the clear idea of release profile of synthesized drug and may prove to modify ADME, duration of action, provide controlled drug release upon hydrolysis in tissue, and finally improve bioavailability.
Further more the synthesized derivatives may also improve the formulation related difficulties. So the synthesized co-drugs may prove to be a good class of stable, long acting, low dose and cost effective antihypertensive drug.
Acknowledgements
We would like to thank the Chairman of Krupanidhi College of pharmacy for him financial support and providing facilities for doing the research work. Our thanks are also due to the Indian Institute of Science Bangalore & Astra Zeneca India limited Bangalore for recording the NMR, IR spectra and also CIPLA, Mumbai for providing gift sample of pure drugs.
Reference
- Jukka L, Juhani H, Topio N. Design and synthesis of a Novel L-dopa -Entacapone Codrug.J Med Chem 2002; 45: 1379-82.
- Howards SM, Mariano L, Salmaso S. Bio org med chem. Letter, 307, 258-69, 2006
- Principle and practice of drug stability by Jen carstensen. 2nd edition. published by Marcel Dekker.
- Brain, Furniss, Anthony J. Determination of physical constants, Determination of melting points.chapter 2.33 in Vogel textbook of practical Organic chemistry, London: ELBS,1989.236-238.
- Indian Pharmacopoeia, published by controllers of India,New Delhi, 4th edition, Vol. 2,1996; 634-35.
- British Pharmacopoeia, Her Majesty’s Stationary Office,London. 2005, 5th edition, Vol. 2, 1777-78.

This work is licensed under a Creative Commons Attribution 4.0 International License.









