Hydrolysis of Nitrile in Presence of NaY Zeolite under Microwave Irradiation
Ravinder Singh* and Ramesh Kumar
Department of Chemistry, Pt. N.R.S. Government College, Rohtak - 124 001 (India).
The hydrolysis of nitriles in presence of NaY zeolite under microwave irradiations gives corresponding amide in high yield in few minutes.
KEYWORDS:Microwave; Amide; Nitrile; Zeolite
Download this article as:| Copy the following to cite this article: Singh R, Kumar R. Hydrolysis of Nitrile in Presence of NaY Zeolite under Microwave Irradiation. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Singh R, Kumar R. Hydrolysis of Nitrile in Presence of NaY Zeolite under Microwave Irradiation. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24880 |
Introduction
Development of the simple and general synthetic routes for widely used organic compounds from the readily reagents is one of the major challenges in organic synthesis. Zeolites have found many important industrial, agricultural applications due to their- High activity levels, regenerability, molecular shape selectivity1-9. Zeolites are effective catalysts in organic chemistry and they have great utility in industry10 in Alkylation reaction, polymerisation, cyclization11, photoreduction12, or preparation of nitroalkenes13, occur in gas phase or with reactants sorbed within zeolite in inert solvent. NaY zeolites has been used as catalyst for number of organic reactions and offer several advantages over other classical catalysts i.e. non-corrosive properties, cheapness, mild reactions, high yields, high selectivity, ease of setting and work up. In the last few years a growing interest has also been shown in the use of microwave irradiation in organic synthesis.14-15
The hydrolysis of nitriles under conventional heating methods is reported in literature16. But this method requires longer reaction time and tadius work up. So, we have the need of alternative technique for the hydrolysis of nitriles.
Now we want to explore our work for the hydrolysis of nitriles using NaY zeolite in presence of microwave irradiations(See scheme 1).. The organic reactions coupled with microwaves undergo completion with in a few minutes, results in an increase in the purity of the products besides enhancing the chemical yields.
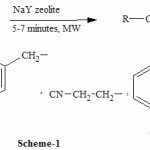 |
Scheme 1 Click here to View scheme |
We started our work by heating 2-Pyridylacetonitrile in presence of NaY zeolite as catalyst under microwave irradiations(See scheme-1) and we obtained corresponding amide in 76% yield in just 5-minutes(See table 1). The product was identified on the basis of their IR, 1H-NMR and by comparison of their Rf values with those of the authentic samples prepared by standard routes.
The hydrolysis of cyanides to corresponding amides occurs on the surface of NaY zeolite catalyst having composition: crystallinity 100%; SiO2 63.80%; Al2O3 22.90%; NaO 13.30%; molar ratio SiO2 / Al2O3: 4.73; specific area (B.E.T) 850 m2 / g; pore volume 0.32 cm3 / g; diameter of crystallite 3.5 mm; diameter of granulate 150 mm; pH of water suspension 10.05.
The hydrolysis of cyanides to corresponding amides most probably involves the co-ordination of cyanides to metal cations of NaY zeolite catalyst through the nitrogen atom which become susceptible to nucleophilic attack at the carbon atom and thus results in enhancement of the rate of nucleophilic attack on nitriles co-ordinated to a metal ion is generally in the range 106 to 108(See scheme 2). The amide needs to be expelled from the co-ordination sphere and be replaced by a new nitrile molecule in order to make a more favourable catalytic system.
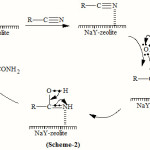 |
Scheme 2 |
In a similar ways, we have also synthesized other amides in presence of NaY zeolite under microwave irradiations in high yield in shorter reaction time(See table 1).
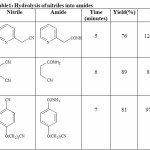 |
Table 1 Click here to View table |
Conclusions
In conclusion, we have shown that the hydrolysis of nitriles into corresponding amides in presence of NaY zeolite as catalyst occurs in a few minutes with improved yield under microwave irradiation.
Experimental section
Typical Experimental Procedure
The suspension of a nitrile (200mg) in water (5ml) in presence of NaY zeolite (800mg) as catalyst is taken in the open erlenmyer flask. The flask was subjected to 70% irradiation level (560W) in the kenstar OM-9925 (800W) unmodified domestic microwave oven operating at 2450MHz for 5-7 minutes. The contents were allowed to cool and poured into water (30ml). The solid separated was filtered off and washed with ethanol (3ml) and recrystallised (from DMF).
2-Pyridylacetamide
Mp 120-122oC. (colorless needles, water). IR (KBr) cm-1: 3377, 3188, 3112, 3017, 1678, 1646, 1597, 1570, 1439, 1402. 1H NMR (CD3OD, 200 MHz): 8.51-8.43 (m, 1H, H-C(6′)), 7.78 (td, J = 7.8, 1.8, H-C(4′)), 7.40 (d, J = 8.0, H-C(3′)), 7.30 (ddd, J = 8, 5, 1, H-C(5′)), 3.75 (s, 2H-C(2)). 13C NMR (CD3OD, 50 MHz): 174.97 (C1), 156.82 (C2′), 149.86 (C6′), 138.76 (C4′), 125.69 (C3′), 123.59 (C5′), 45.12 (C2). Anal. calc. for C7H8N2O (136.06): C 61.75, H 5.92, N 20.57, found: C 62.08, H 5.57, N 20.83.
3-Cyanopropanamide
Mp 86-88oC (colourless amorphous solid). IR (KBr) cm-1: 3414, 3225, 2293, 2248, 1681, 1619,1421. 1H NMR (D2O, 200 MHz) : 2.80-2.65 (m, 4H, 2H-C(2), 2H-C(3)). 13C NMR (D2O, 50 MHz) : 177.90 (C1), 123.04 (C4), 32.38 (C2), 15.12 (C3). MS (CI, isobutane): 197 (2 M++1). Anal. calc. for C4H6N2O (98.11): C 48.97, H 6.16, found: C 49.34, H 6.08.
4-(5-Cyanopentoxy)benzamide
Colourless solid mp 97-101oC. IR (KBr) cm-1: 3466, 2140, 1614, 1566, 1401. 1H NMR (CD3OD,200 MHz): 7.84 (AA’BB’, J = 6.8, 2, H-C(2), H-C(6)), 6.98 (AA’BB’, J = 6.8, 2, H-C(3), H-C(5)), 4.06 (t, J = 6.2, 2H-C(1′)), 2.49 (t, J = 6.8, 2H-C(5′)), 1.90-1.57 (m, 6H). MS (EI, 70 eV): 232 (M+, 25), 137 (33), 121 (100), 96 (14), 55 (8), 41(5). Anal. calc. for C13H16N2O2 (232.12): C 67.22, H 6.94, N 12.06, found: C 67.54, H 6.90, N 12.
Acknowledgements
We thank Professor A.J.Bellamy (Swindon,UK) for inspiration.
References
- Dyer, A.; “An Introduction to Zeolite Molecular Sieves”, (1988) John Wiley & Sons, Inc.
- Barrer, R.M.; J. Chem. Soc., (1950) 2342–2350
- Barrer, R.M.; “Hydrothermal Chemistry of Zeolites”, (1982) Academic Press Inc., London.
- Breck, D.W.; “Zeolite Molecular Sieves”, (1974) John Wiley & Sons, Inc.
- Smart, L.; Moore, E.; “Solid State Chemistry: An Introduction” 1st Edition, (1992) Chapman and Hall
- Maxwell, I.E.; Catal. Today, 1 (1987) 385–413
- R.Gedye,F.Smith,K.Westaway,H.Ali ,L.Baldisera,L.Laberge, J.Rousell, Tetrahedron Lett.27(3),(1986),279.
- 8. R.J.Giguere,T.L.Bray,S.M.Duncan,G.M.ajetich,Tetrahedron,Lett.27(41),(1986),4945.
- A.Loupy,L.Perrex,M.Liagre,K.Burle,M.Moneuse,PureAppl.Chem.73(1),(2001).
- Hölderich, W.; Hesse, M.; Naumann, F. Zeolites: catalysts for organic synthesis. Angew. Chem. Int. Ed. Engl. 1988, 27, 226-246.
- Sen, S. E.; Zhang, Y. Z.; Roach, S. L. Zeolite-mediated cyclization of an epoxide-containing polyene. J. Org. Chem. 1996, 61, 9534-9537.
- Rao, J. V.; Uppili, S. R.; Corbin, D. R.; Schwarz, S.; Lustig, S. R.; Ramamurthy, V. Facial selective photoreduction of steroids: role of zeolites. J. Am. Chem. Soc. 1998, 120, 2480-2481.
- Sreekumar, R.; Padmakumar, S. R.; Rugmini, P. Regioselective reduction of epoxides and conjugated carbonyl compounds using zeolite supported zinc borohydride. Tetrahedron Lett. 1998, 39, 5151-5154.
- (a) Caddick, S. Tetrahedron 1995, 51, 10403. (b) Lidstrom, P.; Tierney, J.; Wathey B.; Westman, J. Tetrahedron 2001, 57, 9225. (c) Lauren, R.; Leporterie, A.; Dubac, J.; Berlan, J.; Lauverie S.; Audhuy, F. M. J. Org. Chem. 1992, 57, 7099.
- (a) Bose, A. K.; Manhas, M. S.; Ganguly, S. N.; Sharma, A. H.; Banik, B. K. Synthesis 2002, 1578. (b) Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacquault, P.; Mathe, D. Synthesis 1998, 1213. (c) Varma, R. S. Green Chem. 1999, 1, 43. (d) Varma, R. S. Clean Prod. Pros. 1999, 1, 132.
- Dragana R.Milic.;Dejan M.Opsenica.;Borivoje Adnadevic and Bogdan A. Solaja useful catalyst for Nitrile Hydrolysis. Molecule 2000, 5, 118-126.

This work is licensed under a Creative Commons Attribution 4.0 International License.









