Azopolyamides-Synthesis and Thermal Studies
T. V. Rajendiran* and A. Jayanthi
Research and Post Graduate Department of Chemistry, Pachaiyappa’s College, Chennai - 600 030 (India).
Article Received on :
Article Accepted on :
Article Published : 05 Mar 2011
Fifteen new azopolyamides have been synthesized from 2,2 1 - dimethoxy – 4, 4 1 – diamino azobenzene [(benzenamine – (3,31 - dimethoxy – 4,4 1 – azo ) bis ] condensed with different diacids / diacid chlorides, following low – temperature solution or phosphorylation or interfacial polycondensation method. These polyamides were characterized interms of viscosity, solubility, UV – Visible, FT – IR and X–ray diffraction studies. Thermal stability of these polyamides was studied using TGA and DTA methods.
KEYWORDS:Synthesis; Characterization; Thermal properties
Download this article as:| Copy the following to cite this article: Rajendiran T. V, Jayanthi A. Azopolyamides-Synthesis and Thermal Studies. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Rajendiran T. V, Jayanthi A. Azopolyamides-Synthesis and Thermal Studies. Available from: http://www.orientjchem.org/?p=11658 |
Introduction
With the impetus derived from aerospace and military industries for high – modulus and high strength fibres, considerable progress has been made in recent years on the synthesis of heat – resistant polymers for special end uses. Among the various classes of thermally stable polymers, polyamides are of great importance1. The azo chromophore in a polymer back bone increases the chain stiffness and imparts colour to the polymer2. Such polymers may, therefore, have potential application as high modulus fibres and high grade pigments of good stability towards light and organic solvents3. In recent years, extensive work has been done by many authors. Nanjan and co – workers 4-6 from this laboratory reported the synthesis, characterization and fibre and / or film studies of azopolyamides, polyimides, poly hydrazides, polyamide – imides and polyesters.
Experimental
2,21– Dimethoxy – 4,41 – diamino azobenzene was synthesized from 2 – methoxy – 4 – nitroaniline (m.p 320oC), yield was 40% 7.
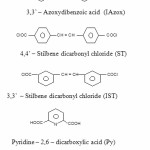
Terephthaloyl chloride (m.p. 79oC), isophthaloyl chloride (43oC), 2,5 – Dichoro terephthalic acid (m.p 306oC), 4, 4 1 – azodibenzoyl chloride (m.p. 164oC), 3,31 – azodibenzoyl chloride (m.p 97 – 98oC), 4,41 – azoxy dibenzoyl chloride (m.p. 146o), 3,31 azoxy dicarbonyl chloride (120oC), 4,41 – stilbene dicarbonyl chloride (m.p. 228 – 232o C), 3,3 – stilbene dicarbonyl chloride (m.p. 228 – 232o C), pyridine – 2,6 – dicarboxylic acid (m.p. 61oC), biphenyl – 4,41 – dicarbonyl chloride (m.p. 184o C), 4,41– dicarboxy diphenyl methane (m.p. 334oC), dibenzyl – 4,41 – dicarbonyl chloride (m.p. 119oC), 4,41 – oxy dibenzoic acid (290oC) and pyromellitoyl diglycine diacid (koch – light GR) were used for synthesis4.
Polymerization
Phosphorylation, low – temperature solution and interfacial polycondersation methods were followed.8-10
Characterization
Viscosity measurements were carried out in conc. H2 SO4 (98%) using ubbelhode viscometer.
UV – Visible spectra of the polymers in conc. H2 SO4 were recorded with a CARL – ZEISS spectrophotometer employing a closed cell. FT – IR spectra of the polymers were recorded using KBr pellets with a Perkin – Elmer 257 or Backman IR 20 spectrometer using KBr pellets.
Non – isothermal studies (TG and DTA) were carried out for all the polymers in nitrogen atmosphere using Stanton – Red craft simultaneous TG – DTA recorder.
Wide angle X – ray Cu Ka radiation (1.541oA) of power 30 Kv and 10 m A was employed.
Results and Discussion
The scheme of polymerization reaction can be given as:
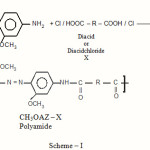 |
Scheme 1 |
 |
Scheme 2 Click here to View scheme |
Scheme I gives the structure and codes of the polyamides. The first four letters of the code refer to the diamine part. The rest of the code refers to the diacid part, based on either the substituent or the rings connecting moiety.
UV – Visible spectral and moisture regain data are presented in Table–1 and appear to depend on (i) the basicity of the diamine (ii) nature of the diacid (iii) initial insolubility of the growing polymer chain in the reaction medium and (iv) on the rigidity of the polyamide.
From Table 1, it is clear that the rigid polymers (para oriented) like CH3 OAZ – TA , CH3OAZ – AZ and CH3O AZ – ST show high viscosity (0.95, 1.20 and 0.80 respectively) since they are structurally rod – like. However, stilbene based polymers show less viscosity on comparison with other two, since during polymerization itself, partial insolubility has been noticed in DMAc / Licl solvent. Very high viscosity (η = 1.20) observed for CH3O AZ – AZ may be due to the fast reaction of amine groups (2 moles) with diacid chloride resulting in high strength / high modulus polymers. Nitrogen atom present in the benzene nucleus of CH3OAZ – Py polymer has no remarkable change or effect on viscosity. Introduction of ether group, azo group or methylene group at the midst of two benzene rings of diacid moiety leads to viscosities of different low orders. Nevertheless, introduction of – N = N – group between the two phenyl groups (CH3O AZ – IAZ) enhances the viscosity to 0.82 which is appreciable compare to other linkages like – CH2 – CH2 – CH2 -, -O – etc. The reason is attributed to the resonance stabilization and trans – isomerism.
Table 1: Properties of Polyamides
|
S. No |
Polymer code |
Polymeri – -zation method |
% Yield |
ηinh* (dlg-1) |
colour |
UV – Visible λmax in nm |
Moisture regain at RH65% |
|
|
UV |
Visible |
|||||||
|
1 |
CH3AZ – TA |
LTS |
90 |
0.95 |
Light brown |
211.4 253.8 |
400 539.4 |
2.80 |
|
2 |
CH3OAZ –IT |
LTS |
80 |
0.65 |
Reddish brown |
209 248.3 |
442 |
2.40 |
|
3 |
CH3OAZ – Cl TA |
LTS,P |
70 |
0.80 |
Cherry red |
212 260 |
468 – |
2.20 |
|
4 |
CH3OAZ – AZ |
LTS,P |
85 |
1.20 |
Dark brown |
202.1 255.2 |
418 540.6 |
1.80 |
|
5 |
CH3OAZ – IAZ |
LTS,P |
75 |
0.60 |
Dark brown |
200.9 340.6 |
395 521.5 |
1.89 |
|
6 |
CH3OAZ – AZOX |
LTS,P |
70 |
0.65 |
Brown |
210.0 265.2 |
– – |
2.00 |
|
7 |
CH3OAZ – IAZOX |
P |
80 |
0.82 |
Dark brown |
205, 245 327 |
– 440 |
1.65 |
|
8 |
CH3OAZ – ST |
LTS, I |
75 |
0.80 |
Light brown |
204 |
348.2 |
2.20 |
|
9 |
CH3OAZ – IST |
LTS |
65 |
0.65 |
Pale yellow |
201 240.4 |
324.0 515.6 |
2.41 |
|
10 |
CH3OAZ – Py |
LTS,P |
60 |
0.70 |
Yellowish red |
262.4 – |
– 490.0 |
2.35 |
|
11 |
CH3OAZ – Diph |
LTS, I |
80 |
0.80 |
Light brown |
208.9 263.3 |
428.0 475.2 |
3.60 |
|
12 |
CH3OAZ – Dipme |
LTS |
85 |
0.76 |
Light brown |
215 270 |
– 515 |
3.15 |
|
13 |
CH3OAZ – Dipheth |
LTS,P |
75 |
0.65 |
Brown |
204.0 338.9 |
375.2 501.4 |
2.85 |
|
14 |
CH3OAZ – Diphoxy |
LTS,P |
70 |
0.74 |
yellowish red |
212.1 242 |
– 455 |
3.40 |
|
15 |
CH3OAZ – Py MDG |
P |
75 |
0.80 |
Dark brown |
230 258.6 |
– 485.0 |
2.90 |
P = Phosphorylation method; I = Interfacial method, LTS – Low temperature solution method.
Table 2: Comparison of viscosity of Azopolyamides 4,5,9
|
S. No. |
Aromatic – aromatic |
Aromatic – aliphatic |
||||
|
Polymer code |
Method |
hinh* (dlg-1) |
Polymer code |
Method |
hinh* (dlg-1) |
|
|
1. |
AZ-AZ
AZ-TA |
LTS36
LTS |
1.48
0.72 |
AZ-MA
AZ-SU AZ-GL AZ-AD |
P P P P |
0.22 0.23 0.34 0.24 |
|
2. |
CH3OAZ-TA
CH3OAZ-AZ CH3OAZ-AZOX CH3OAZ-ST |
LTS LTS LTS LTS |
0.95 1.20 0.65 0.80 |
CH3OAZ-SU
CH3OAZ-AD
|
P LTS P LTS |
0.29 0.36 0.51 0.53 |
|
3. |
CH3AZ-TA
CH3AZ-AZ CH3AZ-AZOX CH3AZ-ST |
LTS LTS LTS LTS |
0.98 2.90 0.54 0.24 |
CH3AZ-MA
CH3AZ-SU CH3AZ-GL CH3AZ-AD |
P P P P |
0.32 0.34 0.44 0.80 |
|
4. |
ClAZ-TA
ClAZ-AZ ClAZ-AZOX ClAZ-ST |
LTS LTS LTS LTS |
0.52 0.64 0.37 0.24 |
ClAZ-MA
ClAZ-SU ClAZ-GL ClAZ-AD |
P P P P |
0.24 0.26 0.38 0.30 |
The – CH2 – linkage lower the viscosity in addition to stability which is evidenced from thermal studies whereas oxygen linkage increases the solubility of the polymers which is useful for spinning fibres or casting films.
It is of interest to compare the CH3, Cl and CH3O substituents on the benzene ring of the diamine part. It has been reported from this laboratory that the order of substituents that favours chains formation is CH3 > O CH3 > Cl. In addition to favouring long chain formation, introduction of CH3 or CH3O or Cl group in the diamine part increases the viscosity compare to unsubstituted polymer (AZ – AZ). Similar comparison can also be made with aromatic – aliphatic polyamides synthesised in this laboratory. Table 2 shows that polymers derived from aliphatic diacids show lesser viscosities. Conclusions may be drawn that wholly aromatic polyamides yield high viscosities compare to aliphatic or easily soluble diacids / diacid chlorides, whatever be the polymerization method followed.
All the polyamides synthesized are soluble in conc. H2SO4 and solubility is slow in CF3COOH, but insoluble in common organic solvents such as CHCl3, C2H5OH and acetone. In other solvents DMAc, DMF, DMSO and NMP, the azopolyamides are soluble to a lesser extent. i.e. partially soluble and in some cases, soluble on heating. Flexible (meta or ortho oriented) polymers are more soluble than rigid (para oriented polymers). This may be due to the higher frequency of incidence of the NHCO groups in the polymers derived from the diacids, which increases the polar nature of the material and lead to higher magnitude in the hydrogen bonding between the polymer and the solvent. Solubility is an important property which determines the extent of processability and end uses of a polymer. Stirring plays little effect on solubility.
The UV – visible values of trans – azobenzene (238.4, 432.9nm), 4, 41 – azodianiline (238, 410 nm), 2,21– dichloro – 4,41– diamino azobenzene (249.6, 457.8nm) and 2,21– dimethyl -4,41– diamins azohenzene (208.07, 265.5 and 407.83 nm) were reported earlier from this laboratory11.
In all the derivatives of trans – azo benzene, there is no appreciable change in visible region because the colour of all the organic compounds are almost dark brown. However, appreciable change in UV values are noticed. π à π* transition easily taking place in the case of 2,21 – dimethoxy – 4,41 – diamino azobenzene. The two methoxy groups throw the two benzene rings out – of – plane due to steric hindrance and consequently reduces the resonance effect through amide linkage and results in slow polymerization reaction which reflects in lesser viscosity.
The gradual change in colour from light to dark brown can be explained on the basis of π electrons transfer from – N = N- towards carbonyl group i.e. conjugation effect is destroyed as evidenced by bathochromic shifts in λ max values. The intensity of the colour varies with pH. In strong bases like 2 M NaOH, the colour of the polymer is intensified. The original colour is, however, restored on the addition of dil. HCl. This may be due to enolisation of the amide group which results in considerable resonance interactions with the –N = N- linkage. Nevertheless, the colour of the diamine and / or polymer depends on the temperature at which it is derived. If the temperature of the reaction mixture is low, the colour is highly golden yellow and, it is brown / dark brown when the temperature is high.
The amide I and II bands in combination with NH stretching are considered to be important for the identification of polyamides. In all the polyamides synthesized, the presence of characteristic bands due to diamines (NH stretching; two bands in the region 3300 – 3500 cm -1) and diacids (C = O stretching 1640 – 1775 cm -1) and (C – H stretching 2500 – 2700 cm -1) confirm the formation of amide linkages and hence of the polyamides. 3300 – 3400 cm -1 frequency shows the presence of NH stretching as broad peaks with medium intensity and indicates that the amides are in trans – form. The broadness of the peaks obtained indicates that the NH groups are involved in hydrogen bonding.
Thermal stability of a polymer depends on the bond energies of the various bonds in the polymer backbone. Higher these bond energies between the atoms, greater is the thermal stability. Further, factors such as high resonance stabilization, high chain stiffness, high degree of crystallinity and high interchain attraction are known to enhance the thermal stability. Physical and morphological factors may also influence the polymer stability.
Table 3: Thermal analysis data of the polyamides (in Nitrogen atm)
|
Polymer Code |
% Weight loss |
T*max (oC) |
AA |
DTA |
|||||||||||||
|
10 |
20 |
30 |
40 |
50 |
60 |
Exotherm temperature |
Endo |
||||||||||
| CH3OAZ–TA |
408 |
480 |
530 |
710 |
800 |
– |
395 |
2 |
365, 570 |
– |
|||||||
| CH3OAZ – IT |
280 |
405 |
485 |
580 |
690 |
830 |
420, 565 |
4 |
350, 460, 520 |
315 |
|||||||
| CH3OAZ –ClTA |
290 |
430 |
502 |
601 |
695 |
844 |
440 |
4 |
360, 440, 510 |
540 |
|||||||
| CH3OAZ –AZ |
410 |
490 |
600 |
– |
– |
– |
565 |
3 |
290, 460 |
340 |
|||||||
| CH3OAZ – IAZ |
275 |
395 |
472 |
582 |
678 |
620 |
560 |
3 |
260, 510 |
258, 315, 545 |
|||||||
| CH3OAZ – AZOX |
330 |
405 |
480 |
625 |
780 |
915 |
475 |
4 |
310, 360, 455, 560 |
310, 400 |
|||||||
| CH3OAZ – IAZOX |
320 |
416 |
492 |
700 |
– |
– |
350 |
2 |
365, 570 |
– |
|||||||
| CH3OAZ – ST |
320 |
400 |
465 |
545 |
760 |
870 |
530 |
4 |
370, 475, 525, 570 |
– |
|||||||
| CH3OAZ – IST |
300 |
390 |
480 |
520 |
720 |
848 |
520 |
3 |
340, 440, 550 |
– |
|||||||
| CH3OAZ – Py |
275 |
396 |
475 |
580 |
675 |
810 |
415, 550 |
4 |
260, 350, 470 |
290 |
|||||||
| CH3OAZ – Diph |
278 |
390 |
474 |
585 |
670 |
770 |
552 |
3 |
290, 495 |
– |
|||||||
| CH3OAZ – DiphMe |
350 |
420 |
505 |
580 |
695 |
– |
320 |
3 |
310, 420 |
575 |
|||||||
| CH3OAZ – Dieth |
280 |
392 |
476 |
585 |
672 |
750 |
542 |
3 |
260, 300, 510 |
– |
|||||||
| CH3OAZ – Diphoxy |
255 |
420 |
500 |
590 |
800 |
– |
380 |
4 |
365, 370, 405, 470 |
– |
|||||||
| CH3OAZ – PyMDG |
340 |
410 |
490 |
570 |
680 |
720 |
305 |
3 |
310, 395, 405 |
520 |
|||||||
Heating rate = 10oC / min
T*max = Temperature at which maximum degradation occurs.
AA = Number of stages of weight loss.
Table 4: Comparison of TGA and DTA data of some polyamides (N2 atm)
|
Polymer Code |
Weight loss % |
Tmax* (oC) |
AA |
Exotherm (oC)§ |
|||||
|
10 |
20 |
30 |
40 |
50 |
60 |
||||
| AZ – AZ**
CH3OAZ – AZ ClAZ – AZ CH3AZ – AZ |
430 410 419 420 |
475 490 468 460 |
– 600 558 635 |
– – 720 – |
– – – – |
– – – – |
450 565 440 370 |
– 3 2 3 |
400@ 290,460 288, 445 165, 370 |
| PA – TA**
PA – IT** CH3OAZ – TA ClAZ – TA CH3AZ – TA |
450 480 408 408 405 |
– – 480 470 480 |
– – 530 550 508 |
– – 710 680 765 |
– – 800 820 – |
– – – – – |
575 585 395 418 365 |
– – 2 2 2 |
– – 365, 570 310, 420 365, 570 |
| ¨AZ – Adi#
CH3OAZ – ST ClAZ – ST CH3AZ – ST |
334 340 280 200 |
361 400 285 440 |
388 502 410 505 |
477 545 546 560 |
550 760 563 800 |
– 870 585 915 |
340 530 300 535 |
– 3 3 3 |
– 370, 475, 525, 570 590 390, 495, 525, 590 |
| CH3OAZ – AZOX
ClAZ – AZOX CH3AZ – AZOX |
330 355 290 |
405 415 400 |
480 528 445 |
625 700 550 |
780 – 780 |
915 – 905 |
475 382 455 |
4 2 4 |
310, 360, 455, 560 340, 390, 485, 530 305, 355, 450, 570 |
AA – No. of stages of weight loss
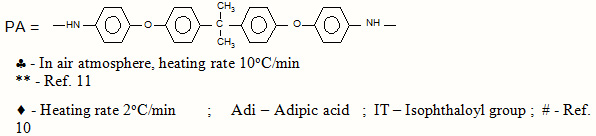
Table 3 summarises the thermal data of all the polyamides obtained in nitrogen atmosphere. Representative TG and DTA curves are presented in Fig. 1. In general, the TG curves show three stages of weight loss. In the first stage, a weight loss of 1-5 % occurs which is accompanied by a small endotherm in the temperature range 100 – 145oC in the DTA curves; this can be attributed to the removal of water or entrapped solvents. The second stage corresponds to a steep fall in the TG curves. In the temperature range 145 – 290o C, there is also a sharp endotherm observed in most of the cases. These may be due to the change in structural pattern of the polymers. The weight loss in this portion is about 20% corresponding to major decomposition of the polymers evidenced by derivative curves. In the third stage, weight loss in the TG curve is steady.
The Tmax values (temperature at which maximum degradation occurs) obtained from DTG (derivative curve of thermogram) correspond to almost 20% weight loss. Greater the molecular weight, greater is the stability and melting point of a polymer. Thus, Tmax is highest for the polymer CH3O AZ – AZ which has the highest molecular weight and it is least for the polymer CH3 OAZ – IST which has the lowest molecular weight. Cross – linking reactions that may occur on heating above 400oC may also contribute to the higher Tmax values observed. The effect of substituents become significant only after 350oC. The low 10% decomposition temperature of CH3OAZ – Diphoxy compared with that of CH3OAZ – Diph may be attributed to the bond nature and the bridging atom present between the two phenyl groups. In this decomposition range, the end groups might react to eliminate water. The poor thermal stability observed for CH3OAZ – PyMDG among the fexible polymers may be due to the fact that the pyridine ring reduces the interchain hydergen bonding considerably leading to less close packed structures. On the other hand, the pyridine nucleus may also pump its lone pair of electrons into the adjacent phenylene rings making them prone to oxidation.
At this juncture, it is reasonable to compare the thermal data of some azopolyamides reported from this laboratory. From Table 4, the order of thermal stability of the polymers, interms of substituents can be given as,
Cl > OCH3 > CH3
which is in the reverse order of viscosity .
DTA curves do not show any melting or glass transitions. Glass transition either occurs in a wide temperature range or masked by the broad endotherms due to escape of volatile impurities. This behaviour can be attributed to the highly rigid nature of the polymers. For the same reason, these polymers are not expected to show any definite melting points since degradation preceeds melting. The number of DTA peaks are almost identical to the number of phases in the TG curves. The peak area in DTA corresponds to enthalpy change in the polyamides. The DTA portion corresponds to this show a number of broad exothermic peaks and hence it corresponds to Tmax in TGA. The exotherms may arise due to decomposition reactions like bond scissions, cyclizations and cross – linking reactions which are quite complex.
Moisture regain
The usage of a fibre is determinded by its tensile properties. Moisture absorption creates loss on tensile strength, particularly in elongation at break. From Table 1, it is seen that the moisture regain values are dependent on the following factors: (i) structure, substituent and coil nature or cavities of the polymer (ii) hydrophilic and hydrophobic nature of the polymer (iii) density or closed packed system of the molecule, number of methylene units and aromatic rings present in the polymers.
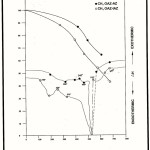 |
Figure 1 Click here to View figure |
Acknowledgement
The authors are thankful to UGC, New Delhi, for financial support under minor Research Projects.
References
- A.H. Frazer, High Temperature Resistant Polymers, Interscinece publishers, John – Wiley&Sons, New York, (1968).
- R.J.W. Lefevere, M.F.O’ Dwyer and R.L. Werner, Aust. J. Chem., 6, 341 (1953).
- F. Agolini and F.P. Gay, Macromolecules, 3, 349 (1970).
- V. Rajendran and M.J. Nanjan, J. Polym. Sci., Polym. Chem. Ed. 25, 829 – 838 (1987); J. Apply. Polym. Sci. 37, 3281 – 3290 (1989).
- V. Rajendran and S.M. Thayumanaswamy, Iran. Polym. J. 11, 4 (2002); J. Appl. Polym. Sci. 93, Issue 3, 1306 – 1316 (2004).
- T.V. Rajendiran and C. Anbuselvan, Asian J. of Chem., 20 (6), 4153 – 4158 (2008).
- O.N. Witt and E. Kopetschini, Ber., 45, 1136 (1946).
- T.V. Rajendiran and Sridevi, Oriental Journal of chemistry 25 (i), 117 – 126 (2009).
- Kallur Sivaraj and M.J. Nanjan, Makromol. Chem., Rapid Commun. 4, 669 – 673 (1983).
- S. Chidambaram and M.J. Nanjan, Makromol Chem. 184 , 2225 (1983).
- M. Balasubramanian, M.J. Nanjan and M.Santappa, Makromol. Chem, 180, 2517 (1979); Makromol, Chem. 182, 5839(1981); J. Appl. Polym. Sci. 27, 1423 (1982).

This work is licensed under a Creative Commons Attribution 4.0 International License.









