Synthesis and Characterization of 4-{4-(2-Methyl-4-Benzylidene-5-Oxo-Imidazol- 1-Yl)Phenyl}-6- (Substitutedphenyl)-5,6-Dihydropyrimidin-2-one and Study of their Antimicrobial Actvities
P. S. Patel1*, R. A. Shah2, D.K. Trivedi2 and P.J. Vyas1
1Department of Chemistry, Sheth L.H. Science College, Mansa - 382 845 (India). 2Department of Chemistry, Sheth M.N.. Science College, Patan - 384 265 (India).
4-{4-(2-methyl-4-benzylidene-5-oxo-imidazol-1-yl)phenyl}-6-(substitutedphenyl)-5,6-dihydropyrimidin-2-one have been prepared by the refluxation for 3 hours of 4-benzylidene-1-{4-[3-(substitutedphenyl)prop-2-enoyl]phenyl}-2-methyl-imidazol-5-one with urea and potassium hydroxide in presence of ethanol . The intermidiate 4-benzylidene-1-{4-[3-(substitutedphenyl)prop-2-enoyl]phenyl}-2-methyl-imidazol-5-one synthesized by the condensation of 1-(4-acetylphenyl)-4-benzylidene-2-methyl-3,5-dihydro-imidazol-5-one with various aldehydes.
KEYWORDS:Synthesis; substituted chalcones; dihydropyrimidin
Download this article as:| Copy the following to cite this article: Patel P. S, Shah R. J, Trivedi D. K, Vyas P. J. Synthesis and Characterization of 4-{4-(2-Methyl-4-Benzylidene-5-Oxo-Imidazol- 1-Yl)Phenyl}-6- (Substitutedphenyl)-5,6-Dihydropyrimidin-2-one and Study of their Antimicrobial Actvities. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Patel P. S, Shah R. J, Trivedi D. K, Vyas P. J. Synthesis and Characterization of 4-{4-(2-Methyl-4-Benzylidene-5-Oxo-Imidazol- 1-Yl)Phenyl}-6- (Substitutedphenyl)-5,6-Dihydropyrimidin-2-one and Study of their Antimicrobial Actvities. Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=23558 |
Introduction
Literature survey reveals that most of the compounds having pyrimidine nucleus possess pharmacological action1-3. A wide spectrum of biological activities like anti-inflammatory,4 antibacterial,5 antifungal,6 antitubercular,7 analgesic and hypothermic8 are found to be associated with compounds having pyrimidine moiety.
Experimental
Melting points were taken in open capillary tube and were uncorrected. IR spectra (KBr) were recorded on I.R. Spectrophotometer of Buck scientific Model No. 500 and instrument used for NMR Spectroscopy was DUL 13C-1, 300 MHz and tetramethyl silane used as internal standard. Solvent used were CDCl3 and DMSO. Purity of the compounds were checked by TLC on silica- G plates. Anti microbial activities were tested by Cup-Borer method.
Preparation of 4-benzylidene-2-methyl-1,3-oxazol-5-one (P-1).
In a 500 ml conical flask equipped with a reflux condenser a mixture of benzaldehyde (39.5g, 0.37M), acetyl glycine (29g, 0.25 M), acetic anhydride (63.5g, 0.62M) and anhydrous sodium acetate (15g, 0.183 M) was placed and heated on an electric hot plate with constant shaking. As soon as the mixture has liquefied completely, transfer the flask to a water bath and heat for 2 hours. Then add 100 ml of ethanol slowly to the contents of the flask, allow the mixture to stand overnight, filter the crystalline product with solution, wash with 25 ml of ice- cold alcohol and then finally wash with 25 ml of boiling water, dry at 100 0C. The yield of almost pure oxazolone was 76 % , m.p. 150 0C.Found: C(70.55%) H(4.82%) N(7.45%) , Calcd. for C11H9NO2: C(70.58%) H(4.85%) N(7.48%).
Preparation of 1-(4-acetylphenyl)-4-benzylidene-2-methyl-imidazol-5-one (P-2)
In a 250 ml conical flask equipped with a reflux condenser a mixture of 4-benzylidene-2-methyl-1,3-oxazol-5-one(18.719g, 0.1M), 1-(4-aminophenyl)ethanone (13.51g, 0.1M), 25 ml pyridine and about one pellet of KOH was placed and was heated on sand bath for 7-8 hours. Then the mixture was poured in ice. The precipitates were collected, washed with 10% HCl and re-crystallized from ethanol. The yield of the product was 72 % and the product melts at 112 0C. Found: C(74.94%)
H(5.26%) N(9.18%) , Calcd. for C19H16N2O2:
C(74.98%) H(5.30%) N(9.20%)
IR (KBr); (cm-1)
3080(= CH-), 2960(-CH Stretch), 1705 (>C=Oimidazolone),1650 (>C=N-), 1600(>C = C<),1375(-CH3bend), 1260(C-N).
Preparation of 4-benzylidene-1-{4-[3-(substitutedphenyl) prop-2-enoyl]phenyl}-2-methyl-imidazol-5-one (P-3).
The solution of 1-(4-acetylphenyl)-4-benzylidene-2-methyl-imidazol-5-one(3.043g, 0.01M) in absolute ethanol (50 ml),substituted benzaldehyde (0.01M) and 2% NaOH (10 ml) were added and reflixed for 10 hours. After refluxing the reaction mixture was concen trated , cooled, filtered and neutralized with dil. HCl. The solid residue thus obtained was recrystallized with suitable solvent.
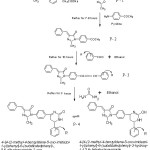 |
Scheme 1 Click here to View scheme |
IR (KBr);P-3a (cm-1)
3080(= CH-), 2900 (-CH Stretch),1725 (>C=Oimidazolone) , 1675(>C=N-), 1590(>C = C<),1375 (-CH3bend), 1260 (C-N), 700(C-Cl).
NMR ;P-3f
δ 2.501 , singlate (3H)(-CH3), δ 3.490, singlate (3H)(-OCH3), δ 5.631, singlate (1H) (=CH-vinylic),δ 6.660-7.902 , multiplate (14H) ( Ar-H)
8.262, singlate(1H) (-OH).
Preparation of 4-{4-[2-methyl-4-benzylidene – 5-oxo-imidazol-1-yl]phenyl}-6-(substituted phenyl)-5,6-dihydropyrimidin-2-one (P-4)
A mixture of 4-benzylidene-1-{4-[3-(substitutedphenyl)prop-2-enoyl]phenyl}-2-methyl-imidazol-5-one(0.01M) ,urea(0.01M) δand 1g. of potassium hydroxide(KOH) in 30ml of ethanol was refluxed for 3 hours. After standing over night the solid for med was collected and crystallised from acetone.
IR (KBr);P-4g (cm-1)
3350(>NH),3240 (-OH), 3090(= CH-) , 2950(-CH Stretch ), 1720 (>C=O ) ,1600 (>C=N-), 1160 (C-N), 1500(>C = C<),1460(-CH2-bend),1400 (-CH3 bend), 1240 (C-O),
NMR ;P-4e
δ 1.123, doublet (2H)(-CH2-), 2.258, triplet (1H)(-CH<), 2.490,singlate (3H)(-CH3), 3.361, singlate (1H)( -NH-), δ 5.416, singlate (1H) (=CH-vinylic), δ 6.588-7.944, multiplate (13H) ( Ar-H), δ 8.097, singlate (1H)(-OH).
Acknowledgments
The authors are thankful to the Sheth L.H.Science College, Mansa for providing research facilities.One of the author Pankaj S. Patel is thankful to UGC. Ganeshkhind, pune.for Teacher Research Fellowship.
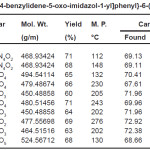 |
Table 1: Physical constant of 4-{4-[2-methyl-4-benzylidene-5-oxo-imidazol-1-yl]phenyl}-6-(substitutedphenyl)-5,6-dihydropyrimidin-2-one Click here to View table |
Table 2: Antimicrobial activities of 4-{4-[2-methyl-4-benzylidene-5-oxo -imidazol-1-yl]phenyl}-6-(substitutedphenyl)-5,6-dihydropyrimidin-2-one
| S. | Comp. | R | Zone of inhibitions in mm | ||
| No. | No. | E.coli | S.aureus | C.albicans | |
| 1 | P-4a | – 4-Cl | 19 | 20 | 22 |
| 2 | P-4b | – 2-Cl | 17 | 18 | 19 |
| 3 | P-4c | – 3-OCH3, -4-OCH3 | 18 | 17 | 18 |
| 4 | P-4d | – 2-NO2 | 15 | 15 | NA |
| 5 | P-4e | – 2-OH | 15 | 15 | 17 |
| 6 | P-4f | – 3-OCH3, -4-OH | 16 | 14 | 19 |
| 7 | P-4g | – 4-OH | 15 | 15 | 14 |
| 8 | P-4h | – 4-N(CH3)2 | 15 | 16 | 18 |
| 9 | P-4i | – 4-OCH3 | 15 | NA | 17 |
| 10 | P-4j | – 3-OCH3, -4-OCH3, -5-OCH3 | 18 | 17 | 20 |
| 11 | Penicillin | – | 18 | 20 | – |
| 12 | Kanamycine | – | 19 | 24 | – |
| 13 | Baycor 25 w.p. | – | – | – | 24 |
| 14 | Amphotericine | – | – | – | 21 |
References
- Dave C V & Shukla M C, Indian J. Chem., 39B: 210 (2000).
- Patel R B. & Chikhalia K H., J. Indian Chem. Soc., 80: 138 (2003).
- Singh A K & Birendra Kumar, Ashain J. Chem., 9(2): 234 (1997).
- Menozzi G. & Filippelli W, Farmaco., 49(2): 115 (1994), Chem. Abstar, 121 (1994) 205269h
- Barot V M., Ashain J. Chem., 8(4): 802 (1996).
- Khan M H. and Nizamuddin, J.Food Agric Chem., 43: 2719 (1995).
- Gangiee A and Adaer G, J. Med. Chem., 42: 2447 (1999).
- Ahluwalia V K and Gupta C, Heterocycles, 32(5): 907 (1991).

This work is licensed under a Creative Commons Attribution 4.0 International License.









