Bio-Mediated Synthesis of Silver Nanoparticles Utilizing Aqueous Leaf Extract of Lantana camera and their Application as Nanophotocatalyst and Antimicrobial Agents
Syed Julkar Nine  , Md. Akkas Uddin, Md. Sabur Ahammad Khan
, Md. Akkas Uddin, Md. Sabur Ahammad Khan  , Arup Kumer Roy
, Arup Kumer Roy  and M. K. Mohammad Ziaul Hyder *
and M. K. Mohammad Ziaul Hyder *
Department of Chemistry, Chittagong University of Engineering and Technology, Chattogram, Bangladesh.
Corresponding Author Email:ziaulhyder@cuet.ac.bd
DOI : http://dx.doi.org/10.13005/ojc/400620
Article Received on : 28 Aug 2024
Article Accepted on :
Article Published : 19 Dec 2024
Reviewed by: Dr. Safoura Rouhi
Second Review by: Dr. Aeed Al-fahdawi
Final Approval by: Dr. Ioana Stanciu
This present study aimed toward the bio-mediated synthesis of silver nanoparticles (AgNPs) by utilizing aqueous leaf extract of Lantana camera with silver nitrate (AgNO3) as the precursor. The color transformation from light yellow to the dark brown of the reaction media was initially inveterate of synthesized silver nanoparticles which further examined by observing the characteristics Surface Plasmon Resonance (SPR) band peak of silver nanoparticles at 458 nm. Presence of any functional groups in silver nanoparticles were determined using Fourier Transformed Infrared (FT-IR) spectroscopy with the stretch of bonds of functional groups. Surface morphology and dispersity of nanoparticles were characterized with a Field-Emission Scanning Electron Microscope (FE-SEM) and the spherical poly dispersed nanoparticles were observed with the particle size in between 15 to 35 nm. The crystallinity and purity of AgNPs were revealed by X-ray Diffraction (XRD) analysis and Energy Dispersive Spectroscopy (EDS), respectively. Photocatalytic activity of AgNPs was observed against Methylene blue (MB) dye under solar light irradiation. The AgNPs efficiently degraded the dye by nearly 95% by the exposure time. The degradation of dye with time was fitted to pseudo-first-order and pseudo-second-order kinetic models where the data best fit the pseudo-first-order kinetic model. Synthesized AgNPs showed moderate anti-microbial activity against four gram-positive and gram-negative bacterial strain while they showed very good activity against two anti-fungal strains.
KEYWORDS:Dye Degradation; Green Synthesis; Methylene Blue; Silver Nanoparticles
Download this article as:| Copy the following to cite this article: Nine S. J, Uddin M. A, Khan M. S. A, Roy A. K, Hyder M. K. M. Z. Bio-Mediated Synthesis of Silver Nanoparticles Utilizing Aqueous Leaf Extract of Lantana camera and their Application as Nanophotocatalyst and Antimicrobial Agents. Orient J Chem 2024;40(6). |
| Copy the following to cite this URL: Nine S. J, Uddin M. A, Khan M. S. A, Roy A. K, Hyder M. K. M. Z. Bio-Mediated Synthesis of Silver Nanoparticles Utilizing Aqueous Leaf Extract of Lantana camera and their Application as Nanophotocatalyst and Antimicrobial Agents. Orient J Chem 2024;40(6). Available from: https://bit.ly/41Ini3Z |
Introduction
Nanotechnology pledges scientific progress in the field of modern research of fabrication, manipulating, and individualizing nanoparticles 1. In the fields of biomedical, electronics, food and health care, drug-gene delivery, the importance of nanotechnology is increasing day by day 1,2. A nanoparticle or discerning particle is usually described as a particle with a diameter of between 1 and 100 nm but is sometimes used for large particles up to 500 nm or for fiber and tubes less than 100 nm in only two dimensions3. These particles have special properties in terms of their very tiny size and a very large and high surface-to-volume ratio which are completely different from their bulk nature of counterparts thus their properties are varied with their morphology. Numerous metals are found in nature but all of them are silver, gold, platinum, and palladium synthesized in nanostructure formed. Staggering physicochemical, electrical, optical, and magnetic peculiarities are shown by noble metal nanoparticles. Due to their extraordinary properties, they are extensively used in various fields. Among noble metal nanoparticles, silver is synthesized widely because of its identical features4. The salient characteristics have made them proper to use in pharmaceutics, agriculture, water detoxification and filtration, air filtration, and dye degradation in the textile industry, catalysis, and chemical industries5. Nanoparticles have some emerging properties such as large surface area, faster rates to equilibrium, less resistance to diffusion, and high adsorption. Because of these properties nanoparticles have gained great importance in the last few decades. Researchers explored the application of silver nanoparticles as a dye-removal agent. Having some supercilious physical, chemical, and biological properties silver nanoparticles are very versatile for implementation in various industries. Silver nanoparticles also possess antibacterial and anti-fungal besides these mentioned characteristics and facilitate their application in wastewater treatment6,7. There are some conventional synthesis processes like physical and chemical methods but these methods have some major drawbacks. Using the physical method to synthesize nanoparticles, a large number of particles can be produced e.g., controlled yield, and product can be gained in powder form with fine particles. However, it has some disadvantages such as being very time-consuming and the large space needed8.
On the other hand, the chemical synthesis method involves toxic and hazardous materials, which is not an environment-friendly method. And also prohibited for medical uses as an application. Because nanoparticles synthesized via this route bind chemicals on their surface2. An eco-friendly synthesis method is necessary to overcome these conventional methods. 9,10. Without any of the toxic and hazardous chemicals or any kind of exterior agents environmentally friendly processes are being developed by the researcher recently as a safe way of approach. As a way of green approach various microorganism-mediated syntheses are done by bacteria, fungi, and plants because of their reducing and capping ability to transform metal compounds into reduced nanoparticles11. Also, cellulosic materials, algae, and yeast are used to synthesize various nanoparticles as a way to approach green synthesis12. Plants parts such as leaves, roots, stems, fruit, flower, and rhizome are used for the synthesis of nanoparticles13,14. Above all, the extract of various parts of the plant is used for the synthesis procedure, because plant extract contains various phytochemicals that act as a capping agent 15. Plant extract consists of various metabolites e.g. alkaloids, terpenoids, amino acids, alcoholic compounds, flavones, flavonoids, proteins, polysaccharides, and phenolic compounds are very much responsible for reducing Ag ions into nanoparticles. However, the mechanism is very unknown of the synthesis of nanoparticles with the parts of plant 16. Some of the phytochemicals in plants act as capping agents and stabilizers. Lantana camara (common lantana) is a species of flowering plant that belongs to the family Verbenaceae and is a genus of about 150 species of herbs 17. Lantana is a native species to tropical and subtropical America by origin. And introduced as a decorative and verge plant to other countries in the world. It spread into various areas such as crop fields, railway tracks, to distributed areas as roadsides and hillsides 18. Thus, the Lantana camera contains essential phytochemicals to synthesize nanoparticles using its leaf extract.
In this study, we attempted to establish a robust and reliable green synthesis method for AgNPs by Lantana camera aqueous leaf extract, which is an eco-friendly, cost effective, and free from any kind of toxic materials. Synthesized silver nanoparticles were subjected to investigate photocatalytic activity against dye and also microbial activity evaluated against bacterial strains and fungal strains.
Experimental
Materials
Silver nitrate (AgNO3) (Merck KGaA, Germany), Deionized water (18 mili-Q), Methylene blue (PT. SMART LAB, INDONESIA), Dimethyl sulfoxide (DMSO) (Germany), Mueller Hinton Agar (MHA) media (HIMDIA, India) and Potato Dextrose Agar (PDA). Leaves of Lantana camera.
Synthesis of Silver Nanoparticles
Fresh leaves of Lantana camera collected from various places of Chattogram hill tracks such as rode side, crops filed side etc. Fresh leaves were splashed many times with deionized water to remove any kind of dirt and contamination. Leaves were dried under shaded conditions avoiding sun drying because sun light may affect the metabolites present in the leaves. Then dried leaves were ground to make a fine powder. To make 10 % (w/v) aqueous leaf extract, 1 g of leaf powder was put into 10 mL of di-ionized water and stirred for 60 minutes at 50 °C. Then the prepared extract was cooled down to room temperature and filtered with Whatman No-1 filter paper and kept at 4 °C for further procedure.
A 5 mM aqueous solution of AgNO3 (precursor) was prepared in dark conditions with di-ionized water. Silver nitrate solution and aqueous leaf extract were slowly mixed considering a ratio of 9:1 while stirring at 60 and 70 °C in dark conditions for 2 hours until the color of the reaction media showed a significant change. And then it was kept for 24 hours at room temperature to acquire complete reduction and nucleation of silver nanoparticles. During the reaction, Ag+ was reduced to Ag0 by metabolites present in the aqueous leaf extract which resulted in the formation of silver nanoparticles (AgNPs).
Characterization
Formation of AgNPs preliminary ensured by observing the physical appearance as color change of reaction media from pale yellow to dark brown. Then, Surface Plasmon Resonance (SPR) band peak from an UV-Visible spectrophotometer (T80+ by PG instrument) was taken for confirmation. FT-IR spectroscopy was employed to investigate the presence of any functional groups. Surface morphology and shape of the AgNPs were evaluated by FE-SEM (JSM-7600F) analysis. Crystalline nature and size were evaluated by XRD (PANalytical) analysis and EDX (JSM-7600F) analysis employed to measure the composition and purity of AgNPs.
Photocatalytic Activity
Synthesized AgNPs were subjected to investigate the photocatalytic activity against organic methylene blue dye. This study was investigated under solar light irradiation. The photocatalytic activity was evaluated against various concentrations of methylene blue dye solutions with different amounts of AgNPs catalysts. At the preliminary stage of this photocatalytic reaction, the mixture of methylene blue dye and silver nanoparticles was stirred in the dark until it reached equilibrium and then continued the reaction under sunlight. The absorbance of methylene blue dye was measured during the reaction at different time intervals at 664 nm using UV-Visible spectrophotometer by taking de-ionized as control. The degradation percentage of dye was calculated with the following Equation (1)
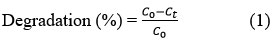
Where C0 = the initial concentration and
Ct = the concentration after time t.
Microbial Activity Assay
Synthesized AgNPs using leaf extract of Lantana camera were subjected to four bacterial strains (two gram-positive and two gram-negative) and two fungal strains to evaluate the microbial activity. Microbial activity assay was examined through Agar disc diffusion method 19 where Mueller Hinton Agar (MHA) media (HIMIDA, India) and Potato Dextrose Agar (PDA) were the basal media for bacterial and fungal strains, respectively. Ceftriaxone and Amphotericin B. were used as standard and Dimethyl sulfoxide (DMSO) was used as control for this study.
Results and Discussion
Silver Nanoparticles synthesis and Characterizations
In the initial step of synthesizing silver nanoparticles (AgNPs), the reaction media turned from a pale yellow to a deep brown, signifying the full reduction of Ag+ (ions) to Ag0 (atoms) and the formation of AgNPs shown in Fig 1. Absorbance measurements taken at various points in time throughout the reaction allowed us to validate the bio-synthesis of AgNPs by revealing a peak at 458 nm in the Surface Plasmon Resonance (SPR) band (Fig 2).
 |
Figure 1: Physical appearance (color change) of reaction media during synthesis of nanoparticle using Lantana camera leaf extract |
 |
Figure 2: UV-Visible spectrum of synthesized AgNPs using Lantana camera (created using Origin Pro 8.5) |
FTIR spectroscopy was employed to elucidate the responsible functional groups for the reduction of Ag from Ag+ those present in the leaf of plant Lantana camera. In (Fig 3) this analysis shows four absorption peaks at
wavenumbers 1615 cm-1, 1510 cm-1, 1209 cm-1, 958 cm-1 corresponding to C=O stretching vibration of carbonyl groups, -NO2 aliphatic nitro group, C-O stretching of the carboxylic group and unsaturated C=C bond, respectively which indicate the presence of these functional bands, a shift in absorption bands of the phytochemical compounds from aqueous extract of Lantana camera as a stabilizing agent.
 |
Figure 3: FTIR spectrum of synthesized AgNPs using Lantana camera (created using Origin Pro 8.5) |
At different magnifications, SEM analysis was conducted to evaluate the surface nature and morphology of plant-mediated produced AgNPs. It displays some nanoparticle morphological characteristics, such as size, shape, and environment showed in the (Fig 4). The spherical nanoparticles in the size range between 15 to 35 nm were observed.
 |
Figure 4: FE-SEM image of synthesized AgNPs using Lantana camera |
Using another spectroscopic analysis, XRD investigation peak pattern of the synthesized AgNPs by the leaf extract of Lantana camara is shown in (Fig 5). The angle of diffraction data was taken for a range 30≤2θ≤80 degrees with a step of 0.01313 degrees. There are four peaks in the diffractogram at 38.15991, 44.10789, 64.54581, and 77.47817, these are the main characteristic peaks for AgNPs. The four diverse diffraction peaks at 38.16°, 44.10°, 64.56° and 77.48° of the 2θ values can be allotted to the planes of 111, 200, 220 and 300 respectively20. By using Debye-Scherrer formula crystalline size and average crystallite size have been estimated from the diffractogram synthesized of AgNPs. Each one of the four peaks was fitted with a Gaussian function to find out the FWHM for the calculation of crystallite size. The FWHM of the fitted Gaussian non-linear curve is taken as FWHM of the peaks in the diffractogram. These values are estimated through the software Origin Pro 8.5. From the data of the four peaks “D” has been calculated. The mean crystalline size of the synthesized silver nanoparticles is found to be approximately 10 nm. Crystallites size and average crystallites size investigated and calculated from XRD data using Scherrer Equation (2) and presented in Table 1.
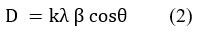
Here, crystal size, D = (nm)
(Scherrer-constant), K = 0.9
(FWHM), β = Full Width at Half Maximum in radians
(wave length of X-ray sources), λ = 0.15406 nm
Peak position, θ = (radians)
 |
Figure 5: X-ray Diffraction peak pattern of synthesized silver nanoparticles (created using Origin Pro 8.5) |
Table 1: Table of Crystalline size calculation from XRD data
|
Peak No |
2θ on x-axis |
FWHM (deg) |
Crystalline size ‘D’ (nm) |
Average crystalline size (nm) |
|
1 |
38.15991 |
0.86833 |
9.68 |
9.95
|
|
2 |
44.10789 |
2.13106 |
4.02 |
|
|
3 |
64.54581 |
0.67211 |
13.98 |
|
|
4 |
77.47817 |
0.83819 |
12.15 |
Elemental analysis of silver nanoparticle was performed using EDX spectroscopy which demonstrated in the (Fig 6). Synthesized AgNPs was confirmed by the intense high signal seen in the characteristics silver (Ag) region of the EDX spectra 21. Metallic synthesized silver nanocrystals exhibit an optical absorption peak at about 3 keV due to the existence of the SPR band. The purity of silver nanoparticles can be described by this analysis hence synthesized silver nanoparticles exhibit high purity. The EDX study provides details on how the aqueous leaf extract of Lantana camara forms pure AgNPs.
 |
Figure 6: EDX analysis of synthesized AgNPs using Lantana camera |
Photocatalytic Activity of AgNPS
Photocatalytic activity of synthesized silver nanoparticles AgNPs were inspected against cationic methylene blue dye under sunlight. The temperature and pH were in optimum condition at 25 °C and 7 respectively during the procedure. This photocatalytic activity was examined against concentrations of methylene blue dye 5 mgL-1. The amount of synthesized AgNPs catalyst used for the degradation of methylene blue in each investigation was 125 mg, 250 mg, and 500 mg per litter of methylene blue solution. At first AgNPs added to methylene blue aqueous solution and continued the reaction in dark until it reaches equilibrium then the reaction media exposed to sunlight hence the degradation of methylene blue continued. During the experiment absorbance of methylene blue dye was observed at 665 nm at different time intervals e.g. 0 min, 30 min, 60 min, 90 min, and 120 min. For the 125 mg, 250 mg, and 500 mg of catalyst against 5 mgL-1 of methylene blue solution, the percentage of degradation was 95.05%, 95.77%, and 95.55% respectively (Fig 7). The degradation of the dye is measured by observing the absorbance at 665 nm, which is gradually decreased as the reaction continues until it reaches equilibrium after 120 min. The same procedure was employed also for 10 mgL-1 of methylene blue dye solution. For 10 mgL-1 solution the degradation efficiency is around 50% with catalyst amounts 125 mg, 250 mg, and 500 mg. Hence the photocatalytic activity of AgNPs exhibited better results against the 5 mgL-1 solution of methylene blue dye.
 |
Figure 7: Absorption spectra (UV-Visible) of dye degradation (a) 125 mg, (b) 250 mg and (c) 500 mg of synthesized AgNPs for 5 mgL-1 concentration, |
Reaction kinetics study
Degradation rate of methylene blue by synthesized AgNPs was investigated through kinetics study (Fig 8)22. To evaluate the kinetics of degradation of methylene blue dye by synthesized AgNPs, the following experiments were carried out for a concentration of 10 mgL-1 of methylene blue at room temperature 25 °C, the amount of catalyst being applied for the procedure was 250 mg per litter of methylene blue solution. The experiment was carried out for 0 min to 120 min when the reaction reached the equilibrium. The kinetics of degradation of methylene blue was investigated with a pseudo-first-order (Equations 3 and 4, Figure 8b) and pseudo-second-order model (Equations 5 and 6, Figure 8c) 23,24.
Pseudo-first-order Eq.
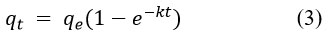
linear form of pseudo first-order Eq.
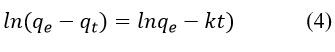
Pseudo-second-order Eq.
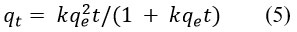
linear form of pseudo second-order Eq.

where qe = concentration of methylene blue at equilibrium and qt = concentration of methylene blue at time (t), respectively, also the co-efficient of each model is listed in the Table 2
The linearity in the pseudo first-order plots fitted better than that of pseudo second-order plots shown in the Fig 8, as confirmed by the higher R2 values. This pseudo first-order kinetic model exhibits the chemical reaction nature of the experiment and its rate of removal of methylene blue dye.
Table 2: Coefficient of kinetics models
|
Material |
Pseudo first-order model |
|
|
K |
R2 |
|
|
AgNPs |
0.018632 |
0.99237 |
|
Material |
Pseudo second-order model |
|
|
K |
R2 |
|
|
AgNPs |
0.001963 |
0.952119 |
 |
Figure 8: Linear plots of (a) Amount of degradation of methylene blue with time, (b) pseudo first-order kinetics and (c) pseudo second-order kinetics of degradation of methylene blue dye by AgNPs. |
Microbial Activity Assay
Two gram-positive strains (S. aures and B. megnaterium), two gram-negative strains (S. typhi and E. coli), and two fungal strains (T. harzianum and A. niger) were tested for in vitro antimicrobial activities against synthesized silver nanoparticles derived from Lantana camara leaf extract. The inhibition zone was assessed using the agar disc diffusion method. Table 3 displays the inhibitory zone execution measured in diameter (mm ± SD). Ceftriaxone and Amphotericin B. were used as standard. The samples were used to evaluate the antimicrobial study S1, S2 and S3 were in different pH e.g. 7, 3, and 12 respectively. S1 and S2 showed activity against all of the microbes gram-positive, gram-negative, and fungal strains whereas S2 which was in pH 3 didn’t show sufficient activity against both gram-positive and gram-negative bacterial strains but showed better activity against two fungal strains TH and AN compared to standard as their inhibition value 20±0.6 and 19.5±0.5 respectively. All samples showed better activity against fungal strains compared to standards. Activities of S1 and S2 were below the inhibition value of standard for gram-positive and gram-negative bacteria shown in the Table 3.
Table 3: Inhibition zone of microbial study of synthesized silver nanoparticles
|
Sample |
Gram-positive |
Gram-negative |
Fungi |
|||
|
|
SA |
BM |
ST |
EC |
TH |
AN |
|
S1 |
13.5±1.0 |
17±0.5 |
17±0.6 |
12±1.0 |
29±1.0 |
23±1.0 |
|
S2 |
– |
– |
– |
– |
20±0.6 |
19.5±0.5 |
|
S3 |
16±1.0 |
12±1.0 |
21±1.0 |
17±0.5 |
16±1.0 |
19.5±1.0 |
|
Ceftriaxone |
38.0±1.0 |
34.0±1.0 |
44.3±0.6 |
40.0±1.0 |
– |
– |
|
Amphotericin B |
– |
– |
– |
– |
17.7±0.5 |
8.3±0.5 |
|
DMSO |
– |
– |
– |
– |
– |
– |
Conclusions
The current work reports the successful biogenic synthesis of silver nanoparticles utilizing an aqueous leaf extract of the wild plant Lantana camera. A sustainable, economical, and non-hazardous chemical-free green synthesis technique has been devised for the synthesis of silver nanoparticles. The photocatalytic and microbiological capabilities of these nanoparticles have been extensively assessed. Synthesized silver nanoparticles were seen to be of reduced dimensions. The inclusion of metabolites presents in the aqueous leaf extract of the Lantana camera served as capping and stabilizing agents during the production of silver nanoparticles, therefore enhancing their versatility in several applications. Under sunlight, the photocatalytic activity of silver nanoparticles was studied, and it was shown that the produced silver particles were capable of degrading methylene blue dye to a great degree. The synthesized silver nanoparticles using Lantana camera exhibited moderate activity against four bacterial strains and excellent activity against fungal strains. The reaction kinetics including the degradation of methylene blue were also investigated. Reaction kinetics of degradation of methylene blue were also investigated which showed best fitting with pseudo first order kinetic model with best correlation value R2 0.99237.
Acknowledgement
This research work was funded from Directorate of Research and Extension (DRE), Chittagong University of Engineering & Technology (CUET) under the research project CUET/DRE/ 2022-23/Chem/024.
Conflict of Interest
The authors declare that they do not posses any known conflicting financial interest.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Malik, S.; Muhammad, K.; Waheed, Y. Molecules. 2023, 28(2), 661.
CrossRef - Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. J. of Nanostructure in Chem. 2019, 9(1), 1-9.
CrossRef - Moodley, J. S.; Krishna, S. B. N., Pillay K., Sershen, Govender P. Adv. in Nat. Sci.: Nanosci. and Nanotech. 2018, 9(1), 015011.
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; et al. J. of nanomat. 2021, 2021(1), 6687290.
CrossRef - Hyder, M. K. M. Z.; Mir, S. H. Inorg.-Org. Comp. for Water and Wastewater Treat. 2022, 2, 65-112.
CrossRef - Marimuthu, S.; Antonisamy, A. J.; Malayandi, S.; Rajendran, K.; Tsai, P. C.; Pugazhendhi, A.; et al. J. Photochem. Photobiol. B: Biol. 2020, 205, 111823.
- Khan, S. A.; Jain, M.; Pandey, A.; Pant, K. K.; Ziora, Z. M.; Blaskovich, M. A.; et al. J. Environ. Manage. 2022, 319, 115675.
CrossRef - Natsuki, J.; Natsuki, T.; Hashimoto, Y. Int. J. Mater. Sci. Appl. 2015, 4(5), 325-32.
CrossRef - Gour, A.; Jain, N. K. Artif. cells, nanomed. and biotechnol. 2019, 47(1), 844-51.
CrossRef - Salem, S. S.; Fouda, A. Biol. Trace Elem. Res. 2021, 199, 344-70.
CrossRef - Yaqoob, A. A.; Umar, K.; Ibrahim, M. N. M. Appl. Nanosci. 2020, 10(5), 1369-78.
CrossRef - Pal, G.; Rai, P.; Pandey, A. Elsevier. 2019, 1-26.
CrossRef - Karthiga, P. Biotechnol. Res. and Innov. 2018, 2(1), 30-6.
CrossRef - Alharbi, N. S.; Alsubhi, N. S.; Felimban, A. I. J. of Radiat. Res. and Appl. Sci. 2022, 15(3), 109-24.
CrossRef - Ahmed, M. J.; Murtaza, G.; Mehmood, A.; Bhatti, T. M. Mater. Lett. 2015;153:10-3.
CrossRef - Krishnaraj, C.; Jagan, E.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.; Mohan, N. Coll. Surf. B. Biointer. 2010, 76(1), 50-6.
CrossRef - Negi, G. C.; Sharma, S.; Vishvakarma, S. C.; Samant, S. S.; Maikhuri, R. K.; Prasad, R. C.; et al. The Bot. Rev. 2019, 85(2), 109-30.
CrossRef - Kato-Noguchi, H.; Kurniadie, D. Plants. 2021, 10(5), 1028.
CrossRef - Balouiri, M.; Sadiki, M.; Ibnsouda, S. K. J. of pharm. anal. 2016, 6(2), 71-9.
CrossRef - Patil, R. B.; Chougale, A. D. Mater. Today: Proc. 2021, 47, 5520-32.
CrossRef - Adebayo-Tayo, B.; Salaam, A.; Ajibade, A. Heli. 2019, 5(10).
CrossRef - Rauf, M.; Ashraf, S. S. Chem. Eng. J. 2009, 151(1-3), 10-8.
CrossRef - Hyder, M. K. M. Z.; Ochiai, B. Microsys. Tech. 2018, 24, 683-90.
CrossRef - Hyder, M. K. M. Z.; Ochiai B. ACS ome. 2022, 7(12), 10355-64.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









