Impact of Graphite on Unsaturated Polyester/Graphite Composites for the Fabrication of Bipolar Plates
M. Karunakaran1,2 , Ravi Subban2*
, Ravi Subban2* , A. Thangamani3
, A. Thangamani3 and Vijayakumar Chinnaswamy Thangavel4
and Vijayakumar Chinnaswamy Thangavel4
1Department of Chemistry, Government Arts College, Udumalpet, Tamilnadu, India.
2Advanced Battery Research Centre, Department of Chemistry, Karpagam Academy of Higher Education: Coimbatore 641021, Tamilnadu, India.
3Department of Chemistry, Karpagam Academy of Higher Education: Coimbatore, Tamilnadu, India.
4Department of Polymer Technology, Kamaraj College of Engineering and Technology (Autonomous), S.P.G.C. Nagar, K.Vellakulam, Tamil Nadu, India.
Corresponding Author E-mail: subbanravi31@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400325
Article Received on : 28 Mar 2024
Article Accepted on : 19 May 2024
Article Published : 05 Jun 2024
Reviewed by: Dr. Spandana Uppuluri
Second Review by: Dr. Naresh Batham
Final Approval by: Dr. Tanay Pramanik
A bipolar plate is one of the most important components of a fuel cell with a proton exchange membrane. Due to the requirement to retain high electrical conductivity, superior mechanical qualities, and low production cost, the development of a suitable material for use as bipolar plate is crucial from a scientific and technological standpoint. Graphite based composites are a viable substitute for metal-based BPs because of their superior mechanical qualities, corrosion resistance, recyclability and cost-effective manufacturing processes. In this work, we attempted to prepare graphite-unsaturated polyester resin composites that would satisfy the technical goals set by the US DOE for 2020 while also making sure that dielectric nature and mechanical strength were well-balanced. Specifically, we tried to investigate how the filler to binder ratio affected the mechanical and dielectric characteristics. With an increase in the amount of graphite up to 5%, the composites' hardness and tensile strength climbed linearly whereas the overall elongation diminishes. The composites' flexural and compressive strengths and the total elongation for compressive and flexural strength increases upto 3% graphite and subsequently drops down as the graphite percentage increases. Composites with 1% graphite have the maximum dielectric strength, while those with 3% graphite show the lowest. Based on these findings, we suggest that a composite that contains between 1% and 3% graphite would work well as a bipolar plate.
KEYWORDS:Dielectric Strength; Graphite, Mechanical Properties; Rooflite; Unsaturated Polyester Resin
Download this article as:| Copy the following to cite this article: Karunakaran M, Subban R, Thangamani A, Thangavel V. C. Impact of Graphite on Unsaturated Polyester/Graphite Composites for the Fabrication of Bipolar Plates. Orient J Chem 2024;40(3). |
| Copy the following to cite this URL: Karunakaran M, Subban R, Thangamani A, Thangavel V. C. Impact of Graphite on Unsaturated Polyester/Graphite Composites for the Fabrication of Bipolar Plates. Orient J Chem 2024;40(3). Available from: https://bit.ly/4e7TxNO |
Introduction
Research on fuel cells for electric vehicles has accelerated due to the growing need for renewable energy sources in the transportation industry. Fuel cells are considered a viable alternative because they offer a more efficient and cleaner source of energy compared to traditional fossil fuels. The most popular fuel cell, the proton exchange membrane fuel cells (PEMFC), is one of the alternative renewable energy sources that can help minimize the harmful consequences of climate change and air pollution brought on by the burning of fossil fuels 1. Solid polymer electrolyte membrane fuel cells, or proton exchange membrane fuel cells, or PEMFCs, are believed to have the highest energy density of all fuel cells 2. Bipolar plates are crucial parts of PEMFCs because they connect the stacks, support the membrane electrode assemblies, collect and conduct electric current, distribute gases like air, hydrogen, and oxygen, evenly, enable proper water management, remove heat, and maintain pressure 3.
Bipolar plates must be addressed since it makes up 40-60% of overall cost and 80% of weight 4-6. Nevertheless, there is a paucity of research on significant factors that might improve the overall efficacy of bipolar plates. The hydrogen fuel cell’s bipolar plates can be constructed from a variety of materials, including metal, carbon polymer composites, and pure graphite. Metal plates are more useful than other materials because of their improved conductivity, lower gas permeability, simplicity of mass production, and lighter and thinner design. However, they fail because of their susceptibility to corrosion in the harsh bipolar plate environment, such as a low pH and water environment7,8,9.
By using non-metal bipolar plates, such as carbon based bipolar plates the weight and corrosive impact of metal based bipolar plates may be decreased. Graphite-based composite plates, which combine superior mechanical qualities and corrosion resistance compared to metals, are beginning to show promise as bipolar plates substitute materials when combined with an appropriate polymer matrix 1. Important considerations to be made include the kind of polymer matrix used, the type of filler used, the dispersion of fillers in the polymer matrix, the surface roughness of the product generated, the necessary molding temperature, the amount of pressure that must be applied, and the appropriate curing time.
Taking into account the arguments above, a review of the literature reveals some of the earlier research on bipolar plates. Bipolar plates made of phenolic resin matrix filled with carbon based materials were described 10. Bipolar plates are made of carbon black (CB), carbon fibers (CF), and multi-walled carbon nanotubes (MWNTs), which are supposedly added to a tiny volume part of graphite composites made with a powdered epoxy resin matrix of the Bisphenol-F type 11. There is a study on composites made of an aromatic polydisulfide resin matrix filled with expanded graphite (EG) at varying expansion ratios that conduct electricity 12. The multiwalled carbon nanotubes (MWCNTs) and graphite powder with polypropylene resin matrix nanocomposite, which were produced by compression molding, were used to form bipolar plates. For these polypropylene composites, single fuel cell stack integration and performance experiments were carried out and reported 13,14. Using a simple hot pressing technique, polyphenylene sulfide and graphite composites for bipolar plates were created, and their mechanical and electrical characteristics were evaluated 15. Based on the literature research, it appears that the carbon polymer composite is a good option and a reliable replacement for the bipolar plate. The US DOE’s (Department of Energy) recommended objective values for mechanical and electrical conductivity, however, are seldom met by them. Therefore, mechanical attributes like hardness, tensile strength, flexural strength, and compressive strength, along with dielectric properties, are examined in relation to the effects of adding graphite to rooflite unsaturated polyester resin in order to create a rooflite resin graphite composite.
Experimental
Reagents
Roof lite unsaturated polyester resin, accelerator, and catalyst, were acquired from S. R. Resins in Coimbatore, Tamilnadu, India. Figure 1 illustrates the synthetic process of creating this resin, which involved condensing propylene glycol, monoethylene glycol, phthalic anhydride, and maleic anhydride at 230°C. The resultant solid 60 % is dissolved in 10 % methyl methacrylate and diluted with 30 % Styrene to decrease the viscosity of the medium. In Fig.1. the synthetic process is displayed with cross link formation between unsaturated polyester chains with either styrene or methyl methacrylate. The technical data sheet for the finished product states that Roof Lite resin has an acid number of 24.0 ± 4.0 mg KOH/gm, a viscosity of 550 ± 100 CPS, and a gel time of 16–24 minutes. 0.2% cobalt naphthanate (6% Co content) was used as an accelerator, and 50% methyl ethyl ketone peroxide (MEKP) combined with hydrogen peroxide catalyzed the resin’s gelling process. Loba Chemicals Private Limited in India supplied extra pure fine powder graphite (98%).
Preparation of Roof lite resin / graphite composites
The composites are made by combining the desired amount of the roof lite resins with cobalt nano-phthalates (1 ml for every 100 g of the resins) and graphite powders in a mechanical agitation process. The dispersion was ultrasonicated for 1 hour at room temperature, degassed, and the required amount of initiator, methyl ethyl ketone peroxide (MEKP) (1 ml per 100 g of resin), was added and rapidly stirred without the formation of air bubbles before being poured into the glass mold. Before pouring the dispersion into the glass mold, the inner surface of the glass plate of the mold was coated with mansion wax polish. The composite was poured into the glass mold, allowed to cure for 24 hours at room temperature, and then post-cured for 2 hours at 80° to 90°C in a hot air oven. Ultimately, the heated mold was permitted to reach room temperature, dried, and the mixture was extracted from the glass mold. Table 1 displays the percentage of graphite added to roof lite resin.
 |
Figure 1: Synthetic process of roof lite unsaturated polyester resin. |
Table 1: Percentage of graphite in samples under this study.
|
Sample code |
Wt. % of graphite added |
|
C1 |
0 |
|
C2 |
1 |
|
C3 |
3 |
|
C4 |
5 |
Methods
The synthesized pure and composite resin plates were tested for hardness. Rockwell cum Brinell Hardness testing machine (M/s Saroj Engineering Udyog PVT LTD, Jaysingpur) was used for testing hardness. The following are the specifications of the machine. The hardness scale chosen in this machine for this study is of category L, which is normally used to test polymeric materials. The ball indentor used for testing the hardness of the samples under study is ¼”, which indicates that this ball indentor is capable of creating an indent of diameter ¼” on the surface of plate specimens of samples tested for hardness. The capacity of this machine is 60 Kg (f) which is 60 Kilogram force. Hardness values were determined at three different points for each plate, and the average of the three values was taken as the actual hardness value of the plate.
The tensile, flexural, and compressive strengths of pure rooflite resin and its composites were determined by using the instrument, Tinius Olsen – Model H50 KL with Horizon software. The maximum load capacity of this instrument is 50 KN. Figure 2 illustrates the configuration that was used to ascertain the tensile strength of pure rooflite resin and its graphite composites. Figure 3 depicts the apparatus used to measure the flexural strength of pure rooflite resin and its graphite composites. The apparatus used to measure the compressive strength of both pure rooflite resin and its graphite composites is shown in Figure 4. The strain rate for both tensile and flexural strength tests is 1 mm/min. The strain rate for the compressive strength test is 0.5 mm/min. The specimens of pure rooflite resin and its composites, used for tensile and flexural strength tests, were of diminution of 100 mm length x 25 mm width. whereas for compressive strength tests, square specimens of the same samples of diminutions 25 mm x 25 mm were used.
 |
Figure 2: The configuration used to determine the tensile strength. |
 |
Figure 3: The 3-point bend test apparatus used to measure the flexural strength. |
 |
Figure 4: The apparatus used to measure the compressive strength. |
The dielectric strength determination comprises the following two parts. a). Determination of breakdown voltage and b. Determination of dielectric strength. The determination of breakdown voltageat which the dielectric resin plates transformed to conducting resin plates was noted as the breakdown voltage, which was measured using high voltage tester at voltages from 0 to 50000 generated by an alternating current (AC) transformer. The unit of breakdown voltage is volts (V).The dielectric strength can be calculated by using the formula
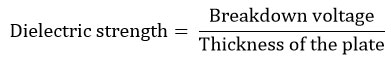
The thickness of the plate used in this formula was the average of thicknesses measured at three different points on the surface of the composite plate. The unit of dielectric strength is V/ mm.
Results and discussion
Hardness analysis
One way to think of hardness is as a measurement of how much plastic deformation a material can withstand when subjected to external stress. Particle reinforcement, such as graphite, boosted the material’s resistance to plastic deformation, thereby increasing its hardness 16. It has been observed that increasing the reinforcement materials concentration and subsequently the Cuffler fiber armament allowed the material’s hardness to increase 17.
Since the measurement of hardness belongs to the surface of the composite material, and it is a surface property. The findings show that sample hardness values are rising in tandem with a raise in the fractional fraction of the supported particles, such as graphite. If the particles are small and the homogeneous distribution is distributed evenly throughout the matrix will increase the hardness of the composite 18. Particle sizes have an impact on hardness values because they are used in small a quantity when compared with matrix resin material, which makes it easier for the particles to penetrate the base material 17. The filler material like graphite which is a solid material is added to the liquid resin to produce the composites. Since the composites are prepared by mixing liquid resin with a small percentage of filler material, the viscosity of the medium will increase gradually. This will permit the graphite particle to penetrate the resin matrix evenly to occupy the interstices present in the resin matrix. If the concentration of filler graphite percentage increases, there is an increase in the viscosity of the medium which will hinder the even distribution of the filler. Due to the increase in the viscosity of the medium, the penetration of the filler in the resin matrix is difficult, which leads to agglomeration of graphite filler, which in turn reduces the hardness of the synthesized composites.
Table 2: Hardness determination results for roof lite resin and its graphite composites
|
Sample Code |
Trial 1 |
Trial 2 |
Trial 3 |
Average Unit ( L) |
|
C 1 |
43.1 |
43.6 |
43.2 |
43.30 |
|
C 2 |
53.4 |
53.4 |
53.8 |
53.53 |
|
C 3 |
74.7 |
74.9 |
74.5 |
74.70 |
|
C 4 |
84.1 |
84.8 |
84.6 |
84.50 |
The hardness of pure roof lite resin and its graphite composites was measured using a Rockwell cum Brinell hardness testing machine as a function of graphite content and the results are given in Table 2 and in Figure 5. From this figure it is clear that the hardness of the roof lite – graphite composites are higher than that of pure roof lite resin. More increasing % of graphite as filler content will increase the hardness of all the synthesized composites. The concentration of graphite used in this study may be below the optimal level which will hinder the even distribution of the filler. One possible explanation for the enhanced hardness properties of graphite could be its large surface area 19. A material’s hardness may be correlated with its intermolecular relationship particularly those between the filler and the polymer matrix. Therefore, the more uniform the dispersion of graphite in the polyester matrix, the higher the hardness 20. Another reason for increasing the hardness of the polyester composites due to the addition of graphite to the polyester matrix, the “interlocking mechanism” with the graphite powder promoted and obstructed the movement of the polymeric chains. Thus, the composites offer greater resistance against external indentation via the hardness tester apparatus, leading to increased hardness measurements 21. In this study composite’s hardness values were elevated. It may be the result of an even dispersion of graphite within the polyester matrix. The even dispersion of graphite assists in avoiding deformation when subjected to external loads 22.
 |
Figure 5: Hardness test results of roof lite resin and its composites. |
Tensile strength
In order for synthetic composite plates used as bipolar plates to withstand the high clamping force of stacking and vibrations during automotive applications, they need to have acceptable mechanical properties 23,24. The tensile test was used to determine the amount of force needed to stretch and elongate the composite until it reaches the break point. Tensile tests were conducted on both the neat polymer and all of the synthesized roof-lite-graphite composites. Typical stress-strain curves obtained for tensile strength determination measurement are shown inFigure6.The curve derived for pure rooflite resin in Figure 6(a). exhibits a linear deformation behavior, wherein the stress increases to a maximum value that indicates yielding before to fracture without plastic deformation25,26,27. As can be observed in Figure 6(b), the curve produced for the rooflite-graphite composite exhibits nonlinear deformation behavior. These curves have a plateau between initial and the final raise. These curves show that all of the specimens, both with and without graphite filler, exhibited brittle behavior. Nevertheless, the strain acquired by all the specimens under examination were around 10.4% at the moment to exhibit brittle behavior, regardless of the samples. The graphite reinforced composites exhibit a bilinear response, according to the experimental results. It is evident that the qualities of the filler utilized affect a composite’s mechanical properties. As observed in the current study, the tensile strength of all composites was found to be lower than that of pristine rooflite resin28. The interfacial contact between graphite and the polymer matrix is essential for improving the mechanical characteristics of graphene composites29. However, in this work, the inclusion of graphite filler results in a reduction in tensile strength, which can be attributed to inadequate graphite interaction within the rooflite resin matrix. J. A. Pandit et al. also offered the argument that the creation of an interfacial connection between the graphite and the rooflite resin matrix may be the cause of the drastic decrease in tensile strength. Moreover, the graphite filler can impede the polymer chains’ ability to move. As a result, the composites shatter instead of dissipating the externally supplied mechanical energy, which lowers their tensile strength value 30. The clustering of the graphite particles causes periodic local stress concentration, which affects the polymer’s inclusive strength31.
 |
Figure 6: Tensile strength analysis – Stress Vs Strain graph for roof lite resin and its composites a) Pure rooflite resin b) Rooflite – graphite composites |
 |
Figure 7: Tensile strength analysis results for roof lite resin and its composites. |
The findings indicate that the tensile characteristics of the neat rooflite were lowered by 76.2% (from 35.2 MPa to 8.37 MPa) upon the addition of 1wt% graphite. This decrease may be explained by the fact that graphite is a brittle substance and that the composite becomes more brittle when it is included into the roof lite resin matrix. The tensile strength reported for general purpose unsaturated polyester resin (GPR) is 42.25 MPa32. In the GPR resin synthesis, the unsaturated polyester sold mass is dissolved in 33 ± 2 % styrene monomer. The styrene is used as solvent for dissolving unsaturated polyester and act as a cross linking agent. In this study, instead of GPR, rooflite resin is used. The unsaturated polyester sold mass is dissolved in 10% methyl methacrylate monomer and 30% styrene monomer during the roof lite resin manufacturing. Here, methyl methacrylate and styrene monomers work together to serve as both a solvent and a cross-linking agent. In this instance, 35.2 MPa is the measured tensile strength for pure rooflite resin. There is a 16.7% decrease in tensile strength when compared with GPR. It may be attributed that the decrease in the tensile strength may be due to the addition of methyl methacrylate monomer. The difference in the molar masses and structures of styrene and methyl methacrylate, may affect the cross linking behavior of the roof lite resin. There by the mobility of the polyester chain may be further restricted. When graphite filler is adds to pure roof lite resin, the graphite distribution in the resin matrix may be affect by the different cross linked patterns is the reason for reduction of tensile strength of the roof lite – graphite composites. Suresha et al., investigation on the impact of micro/nano particles on the mechanical and tribological characteristics of polymer composites revealed a similar pattern33. The increasing the concentration for 1% to 3% or 5% of graphite as filler in the roof lite composite will further reduce the tensile strength and increase the brittleness. Tensile strength values for composites with graphite contents of 3% and 5%, respectively, were determined to be 7.68 MPa and 7.45 MPa for C3 and C4 respectively. The increased concentration of stress regions and the decreased bonding between the matrix and filler are the reasons for this increased brittleness. Higher graphite concentration causes agglomerates to form, which aid in the development of air bubbles and micro cracks when there is a greater interfacial distance between the filler and matrix. Furthermore, if fractures emerge in the composite, the graphite micro particles may function as barriers, making the fracture brittle and producing a less convenient crack route.
Elongation
The tensile analysis findings of the pure resin and its graphite composites demonstrate the elastic properties of the resin and its composites. The elongation at break will give an idea about the material’s elastic nature. High values of elongation at break are often a sign of a very elastic material. The material becomes more brittle, and the matrix loses its elastic characteristics when graphite filler is added. According to the analytical results displayed in Figure 7., elongation at break (%) steadily decreases as filler loading increases. The stiffening of the polymer matrix by the filler is the root cause of the decrease in elongation at break. When the quantity of filler is increased further, a substantial physical link between the filler particles forms, which reduces molecular mobility. This may make the polymer chain stiffer, which would reduce the elongation at break.
Flexural strength analysis
The 3-point bend test according to ISO 178 is used to calculate flexural strength using the Tinius Olsen Model H50 KL with Horizon software. Every flexural test is carried out at room temperature with a fixed strain rate of one millimeter per minute. The stress-strain curve for pure rooflite resin is first displayed in Figure 8(a).In the Figure 8(b).,represents stress-strain curve for rooflite-graphite composites. From the flexural analysis illustrates that a maximum flexural strength of 78.7 MPa was produced by pure rooflite resin with designated sample code C1, whereas rooflite resin-graphite composites with designated sample codes C2 to C4 achieved flexural strength values lower than those of pure rooflite resin. The flexural strength of composite designated as C2 which contain 1% graphite has 26.1MPa. This decrease might be accounted for by the filler material’s high hardness34. This reduction may also be explained by the fact that, the filler is uniformly distributed throughout the polymeric matrix of the composites, which results in high stiffness36,37. This shows that in order to allow mechanical stresses to be passed between the two components, moderate filler amounts are necessary. The composite known as C3 , which contains 3% graphite, has a flexural strength of 31.1 MPa. Good contact between the filler particles and the polymer matrix, as well as the increased rigid characteristics of graphite relative to the polymer matrix, are responsible for the increase in flexural strength. The flexural strength of the composite known as C4, which contains 5% graphite, will drop to 20.3 MPa with a further increase in filler. As the filler quantity increased, the flexural strength began to decrease. It is possible to presume that there is an increase in interfacial adhesion between the filler and matrix, which decreases as the filler concentration increases due to the formation of agglomeration. This filler aggregation and agglomeration increases the amount of voids, which may result in microscopic flaws36. Due to the aggregation of filler in the matrix lead to increase in microscopic structural defects that reduces the performance. It is important to mention that the flexural strength is higher than that of tensile strength. It is because the whole volume of material experience uniform stress while 3-point bend test is performed37. The mechanical properties of rooflite resin composites varied in this work, which is consistent with other research that demonstrated that adding more carbon filler to the polymeric matrix enhanced the mechanical properties and adding even more carbon filler decreased the mechanical properties of the polymer composites 38,39,40. To confirm the mechanical property, the samples developed with the same proportion are tested with flexural strength. In the axial loading, the sample with graphite particle dispersion (3%) has high strength compared to other compositions41. When there is an increase in the dispersion of graphite particle (in the matrix), the brittleness increased. Corresponding fractured surfaces are observed with the help of electron microscope to study. The resin matrix is pulled and detached in the plain composite material. The graphite particle may have super conducting nature and high surface bonding strength. However, while applying the mechanical load, it is does not have superior modulus to resist shear or tear, hence leading to fracture with brittle nature.
 |
Figure 8: Flexural strength analysis – stress Vs strain graph roof lite resin and its composites a) Pure rooflite resin b) Rooflite – graphite composites |
 |
Figure 9: Flexural strength analysis results for roof lite resin and its composites |
Elongation
Elongation at break (%) increases for composites designated as C2 and C3, and decreases for composite designated as C4, as per the results shown in Fig. 9. The reduction in elongation at break is mostly caused by the filler hardening the polymer matrix. It could be explained by the fact that as the filler particle concentration is raised, a strong physical link forms between the particles, reducing molecular mobility. This might minimize the elongation at break by stiffening the polymer chain. Numerous investigations have demonstrated that improving a polymer matrix’s mechanical characteristics is achieved by adding carbon filler. However, there is a decrease in the elongation characteristic beyond a particular filler level, which is dependent on certain matrix-filler systems 42.
Good mechanical qualities are necessary for synthetic composite plates used as bipolar plates in order to survive vibrations and the high clamping strength of mounting in automotive applications. Flexural strength of at least 25 MPa is necessary for a composite to be a bipolar plate, which is one of the many crucial parts of the Proton Exchange Membrane (PEM) multifunctional fuel cell. Based on the findings of this study, it can be concluded that the composite C2 & C3 has a flexural strength greater than 25 MPa. These two plates can therefore be considered as bipolar plates.
Compressive strength analysis
Using Horizon software and the Tinius Olsen Model H50 KL, the compressive strength was ascertained. At room temperature, each compressive test is conducted at a constant strain rate of 0.5 millimeters per minute. First shown in Figure 10(a) is the stress-strain curve for pure rooflite resin. The stress-strain curve for composites made of rooflite and graphite is shown in Figure 10(b). The computed compressive strength and total elongation for pure rooflite resin and its graphite composites are displayed in Figure 11. According to the compressive analysis, pure rooflite resin with sample code C1 produced a maximum compressive strength of 70.5 MPa, while composites of rooflite resin along with graphite with sample codes C2 through C4 produced compressive strength values that were lower than those of pure rooflite resin. The reason why 100% rooflite resin has a greater ultimate strength in compression testing is because it is a solid, hard polymer that weakens as the filler fraction increases along the specimen’s cross section. The composite known as C2, which contains 1% graphite, would exhibit a 74% fall in compressive strength to 18.4 MPa when compared to pure rooflite resin. The increased hardness of the filler material might be the cause of the drop 19. The size of the graphite particles also affects how drastically rooflite resin graphite composites’ compressive strength varies. The compressive strength of the composites increases as the filler graphite content reaches 3%, and drops when the filler concentration reaches 5%. The compressive strength of the composites C3 and C4, which contain 3% and 5% graphite, respectively, is 41.4 MPa and 32.9 MPa. But according to the data depicted in Fig. 11, when compared to pure rooflite resin, the total elongation at break (%) rises for composites labeled as C2 and C3, and falls for composite designated as C4. Perhaps the initial increase in compressive strength was due to the complete dispersal of graphite filler in rooflite resin matrix that allowed uniform distribution of applied compressive load to the dense composite specimen. A possible explanation for the decline in compressive strength of rooflite resin composites with an increase in the % weight of graphite is the inadequate dispersion of the graphite filler within the rooflite resin matrix. Higher filler graphite concentrations might generate fine agglomerates and less bonding between the filler and matrix materials, which lessens the uniform distribution of compressive force43. Compared to other composites with designations C2 and C4, the C3 rooflite resin composite has the maximum compressive strength, making it a material appropriate for use in practical situations where the selected material must be able to handle significant compressive stresses.
 |
Figure 10: Compressive strength analysis –stress Vs strain graph roof lite resin and its composites a) Pure rooflite resin b) Rooflite –graphite composites |
 |
Figure 11: Compression strength analysis results for roof lite resin and its composites |
Dielectric properties
Determination of dielectric strength of rooflite resin and its graphite composite plates
The highest voltage that an insulating material can tolerate before experiencing electrical breakdown, at which point it is electrically conducting, is known as its dielectric strength. The electrical stress that an insulating material experiences is caused by the applied voltage. An insulating substance’s breakdown voltage is the highest applied voltage that results in electrical breakdown of the material. An insulating material undergoes electro-thermal breakdown at higher breakdown voltages as a result of increased leakage current, heat dissipation, and local temperature rise. The breakdown process is irreversible, catastrophic, and caused by an electrochemical collision and corrosion working together. The material near the two test electrodes is punctured and decomposed by the breakdown, which causes an electric spark to be generated that pierces the material’s weak areas and is known as the “treeing effect”44,45. Placing one or more barriers between the two test electrodes, either butting or overlapping, lengthens the time it takes to reach the breakdown voltage and slows the spread of the electrical tree that results from the treeing effect46.
An inherent and crucial characteristic of insulating materials used in the electrical industry, energy storage devices like capacitors, and thin films in high-speed digital electronic circuitry is their dielectric strength. Improved insulation qualities and increased electrical resistance of an insulating material are correlated with higher dielectric strength. A few key characteristics of dielectric or insulating materials are resistivity, which is the resistance to the flow of electric charge, arc resistance, which is the resistance to the flow of electric charge on the surface or through thickness, dielectric strength, which is the maximum voltage before electrical breakdown occurs, dielectric constant, also known as permittivity, which is the ratio of a material’s capacitance to its capacitance of vacuum, indicating how good a dielectric capacitor a material is, and dissipation factor, which is the potential to release thermal energy generated as a result of applied electrical energy.
Table 3: Breakdown voltage and dielectric strength determination of Rooflite resin and its graphite composites
|
Sample code |
Graphite (% weight) |
Breakdown voltage (V) |
Dielectric strength (V/mm) |
|
C1 |
0 |
16,500 |
4342.10 |
|
C2 |
1 |
16,000 |
4597.70 |
|
C3 |
3 |
15,000 |
4297.99 |
|
C4 |
5 |
18,100 |
4444.44 |
 |
Figure 12: Breakdown voltage and Dielectric strength for rooflite resin and its composites |
The dielectric strengths of pure rooflite resin and its synthesized composite plates were measured; the findings are shown in Table 3 and Figure 12. When comparing the breakdown voltage of rooflite resin composites with the designations C2, C3, and pure rooflite resin, the voltage dropped as the proportion weight of graphite increased from 1% to 3% , as shown in Fig. 12. But when the graphite weight is increased to 5%, it hits its maximum. The dielectric strength of the rooflite resin composites named C2 and C4, rose while the composite called C3 decreased when compared to that of pure rooflite resin. The composite material identified as C1 for dielectric strength and C4 for breakdown voltage were the highest values attained by the rooflite resin composite. The material becomes electrically conductive and least resistance at its lowest dielectric strength value. The distance between interfaces, also known as the inter-layer distance, increases when there is less density in the polymeric matrix and less graphite filler present. This results in an enhanced flow of electric charge between the two test electrodes at high electric stress, as demonstrated by the pure rooflite resin designated C2 47. Rooflite resin composite C2 has the maximum dielectric strength and may be chosen as an electrical insulator. The dielectric strength and the breakdown voltage hits its maximum when the graphite weight is increased to 5%. According to percolation theory, high dielectric constant can only be obtained at filler loadings very close to the threshold 48. The filler loading level required to reach high dielectric constant in graphite composites is much lower than that of composites, which enables the graphite composites to possess good mechanical properties. It was observed that as the graphite percentage increases a good number of graphite aggregations were seen in the composite. The dielectric strength of graphite composites is a function of filler loading. The dielectric properties of graphite composites are strongly dependent on the particle size and aggregate structure of the graphite. The dielectric strength of graphite composites is attributed to three effects, i.e. the dispersion of graphite, the polarization effects between isolated graphite aggregates, and the anomalous diffusion within aggregates. In the presence of an electrical field, the charges move inside the graphite aggregates according to the direction of the electrical field in each half cycle and the charges accumulate in the interfacial boundaries between graphite aggregates and the resin, and a dipole moment is imparted to a whole cluster for an isolated which account for the high dielectric strength of the composites.
Conclusion
The synthesized pure and composite resin plates were tested for hardness. The tensile, flexural, and compressive strengths of pure rooflite resin and its composites were determined. Among theunsaturated polyester resin composites prepared with 1,3 and 5% of graphite composite that contains between 1% and 3% graphite exhibited optimum and desirable characteristics and would work well as a bipolar plate. The hardness of the roof lite – graphite composites are higher than that of pure roof lite resin. More increasing % of graphite as filler content will increase the hardness of all the synthesized composites. The tensile strength of all composites was found to be lower than that of pristine rooflite resin and exhibited a bilinear response. Also the elongation at break (%) steadily decreases. The flexural strength values lower than those of pure rooflite resin and elongation at break (%) increases for composites containing 1 and 3% graphite and decreases for composite with 5% graphite. A similar trend was observed when compressive strength was determined. But, compared to other composites with designations C2 and C4, the C3 rooflite resin composite has the maximum compressive strength, making it a material appropriate for use in practical situations where the selected material must be able to handle significant compressive stresses. With respect to the dielectric strength while comparing the breakdown voltage of rooflite resin composites with the designations C2, C3, and pure rooflite resin, the voltage dropped as the proportion weight of graphite increased from 1% to 3%. Rooflite resin composite C2 has the maximum dielectric strength and may be chosen as an electrical insulator. Based on these findings, we suggest that a composite that contains between 1% and 3% graphite would work well as a bipolar plate.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Funding Sources
This research had no external funding.
Author contributions
M.Karunakaran: formal analysis, writing—original draft; Dr. Ravi Subban: —writing— review & editing, supervision, conceptualization; Dr. A. Thangamani — writing—review & editing; Dr. Dr. C. T. Vijayakumar —writing— review & editing, supervision, conceptualization;
Data Availability Statement
The authors confirm that the data supporting the results of this study are available in the article.
References
- Saadat, N; Dhakal, H.N.; Tjong, J.; S. Jaffer, S.; Yang, W.; Sain, M.; Recent advances and future perspectives of carbon materials for fuel cell. Renew. Sustain Energy. Rev. 2021, 138, 110535. https://doi.org/10.1016/j.rser.2020.110535.
CrossRef - Kim, M. ; Choe, J.; Lim, J.W.; Lee, D.G. ; Manufacturing of the carbon/phenol composite bipolar plates for PEMFC with continuous hot rolling process. Compos. Struct. 2015, 132, 1122 -1128. https://doi.org/10.1016/j.compstruct.2015.07.038
CrossRef - Spinelli, L. ; Roncaglia, F. ; Biagi, R.; di Bona, A.; Romagnoli, M.; Mucci, A.Graphite/epoxy composite for building Bipolar Plates. E3S Web Conf. 2022, 334, 04010. https://doi.org/10.1051/e3sconf/202233404010
CrossRef - Boyaci San, F.G. ; Tekin, G.; A review of thermoplastic composites for bipolar plate applications. Int. J. Energy Res. 2023, 37, 283-309. https://doi.org/10.1002/er.3005
CrossRef - Planes, E.; Flandin, L. ; Alberola, N.; Polymer Composites Bipolar Plates for PEMFCs. Energy Procedia. 2012, 20, 311-323. https://doi.org/10.1016/j.egypro.2012.03.031
CrossRef - Bar-On, I. ; Kirchain, R. ; Roth, R.; Technical cost analysis for PEM fuel cells. J. Power Sources. 2002, 109, 71-75. https://doi.org/10.1016/S0378-7753(02)00062-9
CrossRef - Alaswad, A.; Baroutaji, A. ; Achour, H. ; Carton, J. ; Al Makky, A. ; Olabi, A.G. ; Developments in fuel cell technologies in the transport sector. Int. J. Hydrogen Energy. 2014, 41, 16499-16508 https://doi.org/10.1016/j.ijhydene.2016.03.164
CrossRef - Antunes, R.A. ; de Oliveira, M.C.L. ; Ett, G.; Ett, V.; Carbon materials in composite bipolar plates for polymer electrolyte membrane fuel cells: A review of the main challenges to improve electrical performance. J. Power Sources. 2011, 196, 2945-2961. https://doi.org/10.1016/ j.jpowsour.2010.12.041
CrossRef - Pozio, A.; Zaza, F. ; Masci, A. ; Silva, R.F.; Bipolar plate materials for PEMFCs: A conductivity and stability study. J. Power Sources. 2008, 179, 631-639. https://doi.org/10.1016/j.jpowsour.2008.01.038
CrossRef - Mathur, R.B.; Dhakate, S.R. ; Gupta, D.K. ; Dhami, T.L. ; Aggarwal, R.K. ; Effect of different carbon fillers on the properties of graphite composite bipolar plate. J. Mater Process Technol. 2008, 203, 184- 192. https://doi.org/ 10.1016/j.jmatprotec.2007.10.044
CrossRef - Lee, J.H.; Jang, Y.K. ; Hong, C.E. ; Kim, N.H. ; Li, P. ; Lee, H.K. ; Effect of carbon fillers on properties of polymer composite bipolar plates of fuel cells. J. Power Sources. 2009, 193, 523- 529. https://doi.org/ 10.1016/j.jpowsour.2009.04.029
CrossRef - Song, L.N. ; Xiao, M.; Meng, Y.Z. ; Electrically conductive nanocomposites of aromatic polydisulfide/expanded graphite. Compos. Sci. Technol. 2006, 66, 2156 – 2162. https://doi.org/ 10.1016/j.compscitech.2005.12.013
CrossRef - Liao, S.; Yen, C. ; Weng, C.; Lin, Y. ; Ma, C.; Yang, Tsai, M. ; Yen, M. ; Hsiao, M. ; Lee, S.; Preparation and properties of carbon nanotube/polypropylene nanocomposite bipolar plates for polymer electrolyte membrane fuel cells. J. Power Sources. 2008, 185, 1225-1232. https://doi.org/10.1016/j.jpowsour.2008.06.097.
CrossRef - Müller, A. ; Kauranen, P. ; von Ganski, A. ; Hell, B. ; Injection moulding of graphite composite bipolar plates. J. Power Sources. 2006, 154, 467-471. https://doi.org/ 10.1016/j.jpowsour.2005.10.096
CrossRef - Xia, L. ; Li, A. ; Wang, W. ; Yin, Q. ; Lin,H. ; Zhao, Y. ; Effects of resin content and preparing conditions on the properties of polyphenylene sulfide resin/graphite composite for bipolar plate. J. Power Sources. 2008, 178, 363-367. https://doi.org/ 10.1016/j.jpowsour.2007.11.094
CrossRef - Dhanunjayarao, B. ; Sanivada, U.K. ; Swamy Naidu, N. ; Fangueiro, R. ; Effect of graphite particulate on mechanical characterization of hybrid polymer composites. J. Ind. Text. 2022, 51,2594S-2615S. https://doi.org/ 10.1177/15280837211010670
CrossRef - Faten Rashid Al Khalidi, Mustafa Ahmed Rajab, Hardness And Wear Resistance Of Composite Materials Supported By Graphite And Silica Particles And Reinforced By Cuffler Fiber. Am. J. Eng. Res. 2018, 7, 317-322.
- Ali Mutar, M. ; Safi Khliwi, F. ; Jameel kamel, R. ; Mechanical Properties Of new Composite Unsaturated Polyesters Based on Nano Fillers for Marine application. J. Phys. Conf. Ser. 2019, 1294, 052075. https://doi.org/ 10.1088/1742-6596/1294/5/052075
CrossRef - Manjunatha, G. ; Raji George; Nagesh, S.N.; Sridhar, S.B. ; Sunith Babu, L. ; Study of Hardness and Wear Properties of Graphene Based Polyester Resin Composites. Int. J. Sci. Res. Comput. Sci. Eng. Inf. Technol. 2019, 4, 809-811.
- Latief, F.H. ; Chafidz, A. ; Junaedi, H. ; Alfozan, A. ; Khan, R. ; Effect of Alumina Contents on the Physicomechanical Properties of Alumina (Al2O3) Reinforced Polyester Composites. Adv. Polym. Technol. 2019, 1-9. https://doi.org/ 10.1155/2019/5173537
CrossRef - Zhang, X. ; Zheng, J. ; Fang, H. ; Zhang, Y. ; Bai, S. ; He, G. ; Al2O3/graphene reinforced bio-inspired interlocking polyurethane composites with superior mechanical and thermal properties for solid propulsion fuel. Compos. Sci. Technol. 2018, 167, 42-52. https://doi.org/ 10.1016/j.compscitech.2018.07.029
CrossRef - Goyal, R.K.; Kambale, K.R. ; Nene, S.S. ; Selukar, B.S. ; Arbuj, S. ; Mulik, U.P. ; Fabrication, thermal and electrical properties of polyphenylene sulphide/copper composites. Mater. Chem. Phys. 2011, 128, 114-120. https://doi.org/ 10.1016/j.matchemphys.2011.02.065
CrossRef - Kim, J.W. ; Kim, N.H. ; Kuilla, T. ; Kim, T.J. ; Rhee, K.Y. ; Lee, J.H. ; Synergy effects of hybrid carbon system on properties of composite bipolar plates for fuel cells. J. Power Sources. 2010, 195, 5474-5480. https://doi.org/ 10.1016/j.jpowsour.2010.03.083
CrossRef - Hwang, I.U. ; Yu, H.N. ; Kim, S.S. ; Lee, D.G. ; Do Suh, J. ; Lee, S.H. ; Ahn, B.K. ; Kim, S.H. ; Lim, T.W.; Bipolar plate made of carbon fiber epoxy composite for polymer electrolyte membrane fuel cells. J. Power Sources. 2008, 184, 90-94. https://doi.org/10.1016/j.jpowsour.2008.05.088
CrossRef - Ansari, F.; Skrifvars, M. ; Berglund, L. ; Nanostructured biocomposites based on unsaturated polyester resin and a cellulose nanofiber network. Compos. Sci. Technol. 2015, 117,298-306. https://doi.org/ 10.1016/j.compscitech.2015.07.004
CrossRef - Davallo, M.; Pasdar, H. ;. Mohseni, M ; Mechanical Properties of Unsaturated Polyester Resin. Int. J. Chem. Tech. Res. 2010, 2, 2113-2117.
- Feng, L. ; Li, R. ; Yang, H. ; Chen, S. ; Yang, W. ; The Hyperbranched Polyester Reinforced Unsaturated Polyester Resin. Polymers (Basel). 2022, 14,1127. https://doi.org/ 10.3390/polym14061127
CrossRef - Suherman, H. ; Dweiri, R.; Sulong, A.B. ; Zakaria, M.Y. ; Mahyoedin, Y. ; Improvement of the Electrical-Mechanical Performance of Epoxy/Graphite Composites Based on the Effects of Particle Size and Curing Conditions. Polymers (Basel). 2022, 14, 502. https://doi.org/ 10.3390/polym14030502
CrossRef - Mustapha, S.N.H.B. ; Bin Azhman, M.S.A. ; Zakaria, S. ; Roslan, R. ; Mustapha, R.B. ; Chuan, L.T. ; Mechanical Properties of Graphite Filled Unsaturated Polyester and Unsaturated Polyester/Palm Oil Blend Resin. Mater. Sci. Forum. 2020, 981, 150-155. https://doi.org/10.4028/ www.scientific.net/MSF.981.150
CrossRef - Pandit, J.A.; Sudarshan, K. ; Athawale, A.A. ; Electrically conductive epoxy-polyester-graphite nanocomposites modified with aromatic amines. Polymer (Guildf). 2016, 104, 49-60. https://doi.org/ 10.1016/j.polymer.2016.09.084
CrossRef - Divakaran, N. ; Kale, M.B. ; Senthil, T.; Mubarak, S. ; Dhamodharan, D.; Wu, L.; Wang, J. ; Novel Unsaturated Polyester Nanocomposites via Hybrid 3D POSS-Modified Graphene Oxide Reinforcement: Electro-Technical Application Perspective. Nanomaterials. 2020, 10, 260. https://doi.org/10.3390/nano10020260
CrossRef - Al-Mufti, S.M.S. ; Almontasser, A. ; Rizvi, S.J.A. ; Unsaturated Polyester Resin Filled with Cementitious Materials: A Comprehensive Study of Filler Loading Impact on Mechanical Properties, Microstructure, and Water Absorption. ACS Omega. 2023, 8, 20389-20403 https://doi.org/10.1021/acsomega.3c00353
CrossRef - Suresha, B. ; Ravi Kumar, B.N. ; Venkataramareddy, M. ; Jayaraju, T. ; Role of micro/nano fillers on mechanical and tribological properties of polyamide66/polypropylene composites. Mater. Des. 2010, 31, 1993-2000. https://doi.org/10.1016/j.matdes.2009.10.031
CrossRef - Sriram, A. ; Sudharsanan, N. ; Mudhukrishnan, M.; Role of micro/nano fillers on mechanical and tribological properties of polyamide66/polypropylene composites. Int. J. Recent Sci. Res. 2015, 6, 6745-6748.
- Reddy, K.M. ; Vardhan, D.H. ; Reddy, Y.S.K. ; Raghavendra, G. ; Rudrapati, R. ; Experimental Study of Thermal and Mechanical Behaviour of Graphite-Filled UJF Composite. Adv. Mater. Sci. Eng. 2021, 1-7. https://doi.org/10.1155/2021/3739573
CrossRef - Hui, C. ; Hong-bo, L. ; Li, Y. ; Jian-xin, L. ; Li, Y. ; Study on the preparation and properties of novolac epoxy/graphite composite bipolar plate for PEMFC. Int. J. Hydrogen Energy. 2010, 35, 3105-3109. https://doi.org/10.1016/j.ijhydene.2009.08.030
CrossRef - Manta, A. ; Gresil, M. ; Soutis, C. ; Tensile and flexural behaviour of a graphene/epoxy composite: experiments and simulation. J. Phys. Mater. 2020, 3, 014006. https://doi.org/10.1088/2515-7639/ab52d8
CrossRef - Phuangngamphan, M.; Okhawilai, M. ; Hiziroglu, S. ; Rimdusit, S. ; Development of highly conductive graphite-/graphene-filled polybenzoxazine composites for bipolar plates in fuel cells. J. Appl. Polym. Sci. 2019, 36, 47183. https://doi.org/10.1002/app.47183
CrossRef - Gautam, R.K.; Kar, K.K.; Synergistic Effects of Carbon Fillers of Phenolic Resin Based Composite Bipolar Plates on the Performance of PEM Fuel Cell. Fuel Cells. 2016, 16, 179-192. https://doi.org/10.1002/fuce.201500051
CrossRef - Baptista, R. ; Mendão, A. ; Guedes, M. ; Marat-Mendes, R. ; An experimental study on mechanical properties of epoxy-matrix composites containing graphite filler. Procedia Struct. Integr. 2016, 1, 74-81. https://doi.org/10.1016/j.prostr.2016.02.011
CrossRef - Sravanthi, K. ; Mahesh, V. ; Nageswara Rao, B. ; Influence of Carbon Particle in Polymer Matrix Composite over Mechanical Properties and Tribology Behavior. Arch. Metall. Mater. 2021, 66, 1171-1178. https://doi.org/10.24425/amm.2021.136433
CrossRef - Alo, O.A. ; Otunniyi, I.O. ; Pienaar, Hc. ; Development of graphite‐filled polymer blends for application in bipolar plates. Polym. Compos. 41, 3364-3375. https://doi.org/10.1002/pc.25625
CrossRef - Sykam, N. ; Gautam, R.K. ; Kar, K.K. ; Electrical, mechanical, and thermal properties of exfoliated graphite/phenolic resin composite bipolar plate for polymer electrolyte membrane fuel cell. Polym. Eng. Sci. 2015, 55, 917-923. https://doi.org/10.1002/pen.23959
CrossRef - Gautam, R.K. ; Kar, K.K. ; Synthesis and Properties of Highly Conducting Natural Flake Graphite/Phenolic Resin Composite Bipolar Plates for Pem Fuel Cells. Adv. Compos. Lett. 2016, 25, (2016) 096369351602500. https://doi.org/10.1177/096369351602500402
CrossRef - Zayza, M.J.; Jubier, N.J. ; Study The Improvement In The Dielectric Strength of (UPR/MgO) Nanocomposites. Al –Bayaty, S.A. ; IOP Conf. Ser. Mater. Sci. Eng. 2020, 757, 012006. https://doi.org/10.1088/1757-899X/757/1/012006
CrossRef - Vogelsang, R. ; Farr, T. ; Frohlich, K. ; The effect of barriers on electrical tree propagation in composite insulation materials. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 373-382. https://doi.org/ 10.1109/TDEI.2006.1624282
CrossRef - Tanaka, T.; Dielectric nanocomposites with insulating properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914-928. https://doi.org/10.1109/TDEI.2005.1522186
CrossRef - Jianwen Xu, Wong, M. ; Wong, C.P. ; Super high dielectric constant carbon black-filled polymer composites as integral capacitor dielectrics. 2004 Proceedings. 54th Electronic Components and Technology Conference (IEEE Cat. No.04CH37546) , 536. https://doi.org/10.1109/ECTC.2004.1319391
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









