The Suitability of Digestion Techniques in the Assessment of Certain Metals in Standard Reference Materials
1Department of Chemistry, Faculty of Science, King Faisal University, PO. Box 380, Al ahsa, Saudi Arabia.
2Chemistry and Nuclear Physics Institute, Sudan Atomic Energy Commission, P.O. Box 3001, Khartoum, Sudan.
Corresponding Author E-mail: aebrahim@kfu.edu.sa
DOI : http://dx.doi.org/10.13005/ojc/400102
Article Received on : 21 Nov 2023
Article Accepted on : 16 Jan 2024
Article Published : 12 Feb 2024
Reviewed by: Dr. Yakubu Azeh
Second Review by: Dr. Chandrashekara Shekara
Final Approval by: Dr. Tanay Pramanik
The assessment of various digestion methods is of utmost importance in accurately determining the concentrations of elemental metals in soil. In this study, the dry digestion method utilizing a graphite furnace oven was compared to the wet digestion method employing a microwave. Four standard reference materials, namely Randwijk clay, Hengelo sandy soil, Halle sandy soil, and Herveld clay samples, were subjected to analysis using an inductively coupled plasma optical emission spectrometer (ICP-OES). The reliability of the data obtained was ensured by calculating the recovery and error percentage of the results for both digestion methods. The findings indicate that the microwave digestion method is superior for all elements in soil samples, except for calcium (Ca), chromium (Cr), and magnesium (Mg). On the other hand, the dry digestion method may be favored for calcium (Ca), copper (Cu), chromium (Cr), magnesium (Mg), sodium (Na), nickel (Ni), and vanadium (V). A pair t-test statistical analysis was conducted to compare the two methods, revealing significant differences, except for calcium (Ca), chromium (Cr), copper (Cu), iron (Fe), potassium (K), and sodium (Na), indicating a lack of agreement between the two methods, except for these specific elements.
KEYWORDS:Dry digestion; ICP/OES; Microwave digestion; Standard Reference Material
Download this article as:| Copy the following to cite this article: Ahmed A. Y. The Suitability of Digestion Techniques in the Assessment of Certain Metals in Standard Reference Materials. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Ahmed A. Y. The Suitability of Digestion Techniques in the Assessment of Certain Metals in Standard Reference Materials. Orient J Chem 2024;40(1). Available from: https://bit.ly/3uyIz1R |
Introduction
Acidic digestion procedures are employed to convert solid samples into liquid extracts, facilitating the quantification of overall or pseudototal concentrations of metallic elements in soils. This crucial process involves the release of metals from the solid matrix into the acidic solution during extraction. Such procedures are essential for the determination of metals using conventional techniques like inductively coupled plasma optical emission spectrometry or atomic absorption spectroscopy [1]
Numerous acid digestion methods have been documented in the literature for the analysis of heavy metals in soils. These methods encompass a wide range, from mild attacks, such as aqua regia in an open system, to the utilization of hydrofluoric acid in a closed system, which is considered a complete digestion method for the breakdown of silicate matrices [2]. The digestion of samples stands as a major contributor to the uncertainty surrounding analytical results, owing to the significant variations in metal content obtained through different methods [3-6]. To ensure the comparability of data, it is imperative for regulatory agencies to standardize the method employed for determining metal concentrations in soils.
Dlamini et al. [7] presented the optimization, validation, and application of microwave-assisted digestion and inductively coupled plasma mass spectrometry (ICP-MS) for the simultaneous determination of trace metals [boron (B), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), molybdenum (Mo), nickel (Ni), lead (Pb), selenium (Se), vanadium (V), zinc (Zn), and arsenic (As)] in soils from sludgeland.
Naicker et al.[8] described the analysis of twelve trace elements in soil and sediment samples using microwave-assisted and ultrasonic-assisted digestion prior to analysis with inductively coupled plasma optical emission spectroscopy. Agnieszka, et al.[9] conducted a study in which they presented the findings of their investigation into the measurement of heavy metal concentration in soil. They employed two distinct methods for adding soil components into solution and utilized different laboratory techniques and types of measuring equipment. The first method utilized was the hot digestion of soil samples with a mixture of concentrated HNO3 and HClO4, following the prior ashing of organic matter (referred to as the IUNG method). The second method involved a two-stage decomposition process, whereby soil samples were initially hot digested with an oxidizing acid (HNO3) and subsequently with a non-oxidizing acid (HF) (referred to as the two-stage decomposition method). The concentrations of selected heavy metals (Cr, Cu, Fe, Mn, Ni, Pb, and Zn) were determined in solutions obtained through both digestion methods.
The aim of this investigation is to assess the efficacy of two digestion methods, specifically the dry digestion method and the wet digestion method, in the determination of heavy metals in four standard reference materials.
Materials and Methods
The development of the digestion methods and the analysis of four certified reference materials (CRMs), namely clays and sandy soil, were undertaken. HCl and HNO3 were analytical grade reagents and were used as received.
Samples
Four certified reference materials (CRMs) were procured for the purpose of this study. The specific details of these samples are provided below (Table 1).
Table 1: Sample details
|
Sample No. |
Type |
Country |
|
1 |
Clay from river basin |
Randwijk/ Netherland |
|
2 |
Sandy soil |
Hengelo /Netherland |
|
3 |
Sandy soil |
Halle/ Netherland |
|
4 |
Clay |
Herveld/ Netherland |
Two digestion methods were utilized, namely graphite furnace digestion (dry ashing) and microwave digestion, as outlined by Greenberg et al.[10].
Dry digestion
A quantity of 1.00 g of each CRM was weighed and placed into a 30 ml porcelain crucible. The crucible was then introduced into a muffle furnace and gradually heated to a temperature of 700°C, allowing the sample to ashed for a duration of 4 h. Following this, the crucible was carefully removed and cooled in a desiccator, as described by Chattopadhyay et al.[11].
Subsequently, 10 ml of Aqua Regia solution (consisting of a 3:1 v/v ratio of HCl to HNO3) were added to the cooled ash and stirred until dissolved. Any undissolved fraction was allowed to precipitate and then filtered into a 25 ml volumetric flask. The resulting solution was diluted with deionized water to a final volume of 50 ml.
Microwave Digestion
For this method, a mass of 0.2 g of each sample was weighed and placed into a microwave Teflon vessel. The vessel, along with the sample, was inserted into the HTC safety shield. Subsequently, 3.8 ml of HNO3(65%), 5 ml of HCl (37%), 1 ml of HF 40%, and 5 ml of H3BO3 5% were added to the Teflon vessel. The vessel was then sealed and inserted into the rotor segment, which was in turn introduced into the microwave cavity and connected to the temperature sensor. The total digestion method was loaded and, upon completion of the program, the rotor was cooled using water until the solution reached room temperature. The vessel was then opened and the solution transferred into a 100 ml volumetric flask, following the protocol outlined by Ahmed et al. [12].
Sample Analysis
The prepared samples from section 2 were subjected to analysis using inductively coupled plasma optical emission spectrometry (ICP/OES), employing the specified operational conditions showed in Table 2.
Table 2: ICP-OES 725 E Operating conditions
|
Parameter |
Setting |
|
Power Plasma flow Aux. Flow Neb. Flow Replicate read time(s) Sample Uptake time Rinse Time Pump rate Instrumental stabilization delay |
1.2 KW 15 L/min 1.5 L/min 0.75 L/min 10 Sec. 30 Sec. 25 S 15 rpm 15 S |
Results and Discussion
The accurate determination of heavy metals in soils holds significant importance in the process of remediation of contaminated soils and the monitoring of land application of nonhazardous materials containing metals. Prior to the measurement of metal concentrations in soils, sample digestion is often required [13].
Quality control of the two digestion methods
In general, the microwave digestion method applied to all four SRMs yielded accurate results (80-120% Recovery) for all elements, except for Mg and Cr in the Hengelo sandy soil and Herveld clay samples [14].
In comparison to microwave digestion, the dry digestion method demonstrated accurate results for Ca, Cu, K, Mg, Na, and V in the Randwijk clay sample (Table 3), Na, Ni, and V in the Hengelo sandy soil (Table 4), Cr and Cu in the Halle sandy soil (Table 5), and Cu in the Herveld clay.
Table 3: Analysis of Randwijk clay sample
|
Method A: Dry digestion |
||||
|
Element |
Analytical value |
Certified value |
Recovery% |
Error% |
|
Ca |
8391.75 |
7964 |
105.36 |
5.4 |
|
Cr |
133.458 |
98.3 |
135.76 |
35.77 |
|
Cu |
23.9816 |
24.98 |
109 |
4 |
|
Fe |
42183.76 |
33470 |
126 |
26 |
|
K |
17197.8 |
19409 |
88.5 |
11.4 |
|
Mg |
7837.023 |
7185 |
109 |
9 |
|
Mn |
1626.026 |
1244 |
130 |
30.7 |
|
Na |
5258.705 |
6315 |
83.27 |
16.7 |
|
Ni |
55.23023 |
43.62 |
126.6 |
26.6 |
|
V |
75.45993 |
92.63 |
81 |
18 |
|
Zn |
140.8378 |
105.5 |
133 |
33 |
|
Method B: Microwave digestion |
||||
|
Element |
Analytical value |
Certified value |
Recovery% |
Error% |
|
Ca |
6484 |
7964 |
81.4 |
18.6 |
|
Cr |
82.639 |
98.3 |
84.1 |
15.9 |
|
Cu |
21.522 |
24.98 |
86.2 |
13.8 |
|
Fe |
39324 |
33470 |
117.5 |
17.4 |
|
k |
20807.2 |
19409 |
107.2 |
7.2 |
|
Mg |
7294.99 |
7185 |
101.5 |
1.5 |
|
Mn |
1430 |
1244 |
114.9 |
14.9 |
|
Na |
5815 |
6315 |
92.1 |
7.9 |
|
Ni |
38.34 |
43.62 |
87.9 |
12.1 |
|
V |
84.16 |
92.63 |
90.9 |
9.1 |
|
Zn |
86.9 |
105.5 |
82.4 |
17.6 |
From the data presented in Table 3, it can be observed that method A, also known as the Dry digestion method, exhibits favorable outcomes in terms of recovery and error percentage for the elements Ca, Cu, K, Mg, Na, and V. Conversely, it demonstrates unsatisfactory recovery and high error rates for the remaining elements.On the other hand, method B, referred to as the Microwave digestion method, demonstrates commendable recovery and acceptable error rates for all the elements under investigation.Analyzing the dry digestion of the Hengleo soil sample, as illustrated in Table 4, it becomes evident that the dry digestion method proves to be effective in terms of recovery and acceptable error only for Na, Ni, and V. Conversely, the microwave digestion method exhibits satisfactory recovery and error rates for all the elements being studied, with the exception of Mg.
Table 4: Analysis of a sandy soil sample obtained from Hengelo.
|
Method A: Dry digestion |
||||
|
Element |
Analytical value |
Certified value |
Recovery% |
Error% |
|
Ca |
1629.72 |
1238 |
131.6 |
31.6 |
|
Cr |
24.572 |
36.06 |
68.1 |
31.8 |
|
Cu |
11.9733 |
8.424 |
142.133 |
42.13 |
|
Fe |
3091.59 |
2541 |
121.6 |
21.6 |
|
K |
3930.79 |
6382 |
61.5 |
38.4 |
|
Mg |
498.404 |
339.7 |
146.7 |
46.7 |
|
Mn |
93.7402 |
75.93 |
123.4 |
23.4 |
|
Na |
2019.75 |
2262 |
89.2 |
10.7 |
|
Ni |
11.6374 |
11.5 |
101.1 |
1.2 |
|
V |
12.5985 |
11.17 |
112.7 |
12.8 |
|
Zn |
25.407 |
19.35 |
131.3 |
31.3 |
|
Method B: Microwave digestion |
||||
|
Element |
Analytical value |
Certified value |
Recovery% |
Error% |
|
Ca |
1322 |
1238 |
106.7 |
6.9 |
|
Cr |
37.066 |
36.06 |
102.8 |
2.8 |
|
Cu |
7.88 |
8.424 |
93.5 |
6.5 |
|
Fe |
2740 |
2541 |
107.8 |
7.8 |
|
K |
6283.27 |
6382 |
98.5 |
1.5 |
|
Mg |
245.915 |
339.7 |
72.4 |
27.6 |
|
Mn |
68.16 |
75.93 |
89.8 |
10.2 |
|
Na |
1922 |
2262 |
85 |
15 |
|
Ni |
12.9 |
11.5 |
112.2 |
12.2 |
|
V |
10.37 |
11.17 |
92.8 |
7.1 |
|
Zn |
21.16 |
19.35 |
109.4 |
9.3 |
Table 5 presents the quality control outcomes for the Halle sandy soil sample. It is evident from method A that the dry digestion method in the Halle sandy soil sample yields favorable recovery and error rates only for Cr and Cu. In contrast, the microwave digestion method (Method B) demonstrates good recovery and error rates for all the elements under investigation.For the Herveld clay sample, the quality control results are displayed in Table 6. In this case, the dry digestion method (method A) exhibits satisfactory recovery and error rates solely for Ca and Cu. On the other hand, the microwave digestion method (method B) demonstrates good recovery and error rates for all the elements under scrutiny, with the exception of Cr and Mg.
Overall, the quality control results for both the dry digestion method and the microwave digestion method indicate that the latter offers superior recovery and error rates. The ashing process in the dry digestion method is challenging to regulate and presents the potential for uneven heating and cross-contamination of samples [15].
Table 5: Analysis of Halle sandy soil sample
|
Method A: Dry digestion |
||||
|
Element |
Analytical value |
Certified value |
Recovery% |
Error% |
|
Ca |
2876.87 |
2038 |
141.2 |
41.8 |
|
Cr |
53.4526 |
45.69 |
116.9 |
16.9 |
|
Cu |
12.2088 |
12.58 |
97 |
2.9 |
|
Fe |
5362.1 |
3972 |
135 |
35 |
|
K |
6489.39 |
8848 |
73.3 |
26.7 |
|
Mg |
709.971 |
505.9 |
140.3 |
40.3 |
|
Mn |
315.745 |
231.1 |
136.6 |
36.6 |
|
Na |
2835.9 |
3710 |
76.4 |
23.6 |
|
Ni |
11.1007 |
8.008 |
138.75 |
38.75 |
|
V |
39.6215 |
26.83 |
147.7 |
47.7 |
|
Zn |
37.6768 |
30.33 |
124.2 |
24.2 |
|
Method B: Microwave digestion |
||||
|
Element |
Analytical value |
Certified value |
Recovery% |
Error% |
|
Ca |
1960 |
2038 |
96.1 |
3.8 |
|
Cr |
42.509 |
45.69 |
93 |
6.9 |
|
Cu |
11.732 |
12.58 |
93.2 |
6.7 |
|
Fe |
4307 |
3972 |
108.4 |
8.4 |
|
K |
8487.72 |
8848 |
95.9 |
4.1 |
|
Mg |
407.893 |
505.9 |
80.6 |
19.4 |
|
Mn |
232.9 |
231.1 |
100.8 |
0.7 |
|
Na |
3149 |
3710 |
84.9 |
15.1 |
|
Ni |
8.444 |
8.008 |
105.4 |
5.4 |
|
V |
24.42 |
26.83 |
91 |
8.9 |
|
Zn |
27.84 |
30.33 |
91.8 |
8.2 |
Table 6: Analysis of Herveld clay sample
|
Method A: Dry digestion |
||||
|
Element |
Analytical value |
Certified value |
Recovery% |
Error% |
|
Ca |
3886.53 |
4030 |
96.4 |
3.6 |
|
Cr |
58.73899 |
79.91 |
73.1 |
26.9 |
|
Cu |
16.24053 |
13.8 |
117.4 |
17.4 |
|
Fe |
14545.17 |
19380 |
75.1 |
24.9 |
|
K |
10711.5 |
15895 |
67.4 |
32.6 |
|
Mg |
1926.547 |
3070 |
62.7 |
37.2 |
|
Mn |
404.6386 |
603.1 |
67.1 |
32.9 |
|
Na |
4731.833 |
6985 |
67.7 |
32.3 |
|
Ni |
12.76765 |
19.58 |
65.2 |
34.8 |
|
V |
32.19403 |
51.55 |
62.4 |
37.5 |
|
Zn |
91.21643 |
123 |
74.1 |
25.9 |
|
Method B: Microwave digestion |
||||
|
Element |
Analytical value |
Certified value |
Recovery% |
Error% |
|
Ca |
3549 |
4030 |
88.1 |
11.9 |
|
Cr |
59.308 |
79.91 |
74.2 |
25.8 |
|
Cu |
12.289 |
13.8 |
89 |
11 |
|
Fe |
19520.9 |
19380 |
100.7 |
0.7 |
|
K |
14186.53 |
15895 |
89.3 |
10.7 |
|
Mg |
2326.479 |
3070 |
75.8 |
24.2 |
|
Mn |
598.5 |
603.1 |
99.2 |
0.76 |
|
Na |
6021 |
6985 |
86.2 |
13.8 |
|
Ni |
19.37 |
19.58 |
98.9 |
1.1 |
|
V |
48.15 |
51.55 |
93.4 |
6.6 |
|
Zn |
107.1 |
123 |
87.1 |
12.9 |
Comparison between the dry and microwave digestion methods
To conduct a comparison between the dry digestion and microwave digestion methods employed for the preparation of four Standard Reference Materials (SRMs) in order to determine the concentration of eleven elements using Inductively Coupled Plasma Optical Emission Spectrometry (ICP/OES), a paired t-test was used. The paired t-test was implemented using the equations provided by Shigeki Tsuneyaet al. [16], denoted as equation 1 and equation 2.
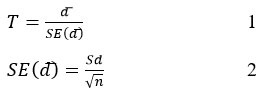
Where: T represents the calculated t-value, d͞ represents the mean difference, Sd represents the standard deviation of the difference, SE(͞d) represents the standard error of the mean difference, and N represents the number of readings.
Consequently, the paired t-test results for the dry and microwave digestion methods were obtained for the four SRMs using equations 1 and 2. These results were then tabulated in Tables 7-10, which display a comparison between the dry and microwave digestion methods.
Table 7: Randwijk clay sample comparing the dry and microwave digestion methods by mean of paired t-test.
|
Element |
Difference(µg /g) |
standard deviation(µg/g) |
SEof the mean difference |
Calculated t |
|
Ca |
5907.75 |
4983.218 |
1502.497 |
-0.38129 |
|
Cr |
1050.819 |
4500.089 |
1356.828 |
-0.42222 |
|
Cu |
215.4596 |
4954.276 |
1493.77 |
-0.38351 |
|
Fe |
2859.76 |
5212.005 |
1571.479 |
-0.36455 |
|
K |
-13609.4 |
5355.932 |
1614.874 |
-0.35475 |
|
Mg |
542.032 |
2850.27 |
859.3888 |
-0.66661 |
|
Mn |
1196.026 |
3077.201 |
927.811 |
-0.61745 |
|
Na |
-4556.3 |
3342.704 |
1007.863 |
-0.56841 |
|
Ni |
46.89023 |
2708.525 |
816.651 |
-0.7015 |
|
V |
-8.70007 |
3054.849 |
921.0715 |
-0.62197 |
|
Zn |
53.9378 |
3574.414 |
1077.726 |
-0.53156 |
|
|
d̅=-572.88 |
|
||
As demonstrated in Table 7, and based on the tabulated values for the two-sided t-distribution, the values for t0.05 (t=1.8) and t 0.025 (t=2.2) exhibit no significant disparity. This indicates that both procedures are in concurrence with one another.
Table 8: Hengelo sandy soil sample comparing dry and wet digestion by mean of paired t-test.
|
Element |
Difference(µg/g) |
standard deviation(µg/g) |
SEof the meandifference |
Calculated t |
|
Ca |
707.7238 |
840.9866 |
253.567 |
-0.07477 |
|
Cr |
-12.494 |
806.0094 |
243.021 |
-0.07801 |
|
Cu |
16.09332 |
849.23 |
256.0525 |
-0.07404 |
|
Fe |
351.587 |
899.8386 |
271.3116 |
-0.06988 |
|
K |
-2352.48 |
944.4462 |
284.7612 |
-0.06658 |
|
Mg |
952.4885 |
355.1257 |
107.0744 |
-0.17706 |
|
Mn |
25.58015 |
41.45191 |
12.49822 |
-1.5169 |
|
Na |
97.74583 |
8940.762 |
2695.741 |
-0.00703 |
|
Ni |
-1.26257 |
8947.76 |
2697.851 |
-0.00703 |
|
V |
2.228467 |
8947.618 |
2697.808 |
-0.00703 |
|
Zn |
4.246995 |
10617.98 |
3201.443 |
-0.00592 |
|
|
d̅=-18.96 |
|
||
As depicted in Table 8, and based on the tabulated values for the t-distribution with two tails, it can be observed that for t0.05 (t=1.8) and t0.025 (t=2.2), there is no statistically significant difference between any of the elements. This implies that both methods are in agreement with each other.
Table 9: Comparison between dry and wet digestion of Halle sandy soil, utilizing the paired t-test as the statistical measure.
|
Element |
Difference(µg/g) |
Standard deviation(µg/g) |
SEof the meandifference |
Calculated t |
|
Ca |
21916.87 |
8475.886 |
2555.576 |
1.497961 |
|
Cr |
10.94358 |
6012.146 |
1812.73 |
2.111816 |
|
Cu |
0.476819 |
6291.67 |
1897.01 |
2.017993 |
|
Fe |
19055.1 |
6613.085 |
1993.92 |
1.919912 |
|
K |
-3998.33 |
2611.752 |
787.4727 |
4.861314 |
|
Mg |
4422.078 |
1974.002 |
595.184 |
6.43188 |
|
Mn |
632.8446 |
1539.768 |
464.2575 |
8.245752 |
|
Na |
-313.101 |
1549.149 |
467.0861 |
8.195818 |
|
Ni |
18.65667 |
1515.711 |
457.0042 |
8.376623 |
|
V |
15.20146 |
1513.387 |
456.3034 |
8.389489 |
|
Zn |
348.9279 |
1622.398 |
489.1713 |
7.82579 |
|
|
d̅=3828.15 |
|
||
As evidenced by the data presented in Table 9 and the corresponding values for the two-sided t-distribution, it is noteworthy that t0.05 (t=1.8) and t0.025 (t=2.2) exhibit statistically significant disparities across all variables, with the exception of Ca. This discrepancy implies that the two methods employed do not concur, except in relation to Ca.
Table10: Herveld clay sample comparing dry and wet digestion by mean of paired t-test.
|
Element |
Difference(µg/g) |
Standard deviation(µg/g) |
SEof the mean difference |
Calculated t |
|
Ca |
337.5301 |
2294.337 |
691.7686 |
-0.94701 |
|
Cr |
-28.569 |
2270.791 |
684.6691 |
-0.95683 |
|
Cu |
42.95153 |
2380.47 |
717.7387 |
-0.91275 |
|
Fe |
2592.265 |
2504.446 |
755.1188 |
-0.86756 |
|
K |
-6475.03 |
2279.051 |
687.1597 |
-0.95337 |
|
Mg |
-399.932 |
1028.625 |
310.1421 |
-2.1123 |
|
Mn |
-293.861 |
1121.838 |
338.247 |
-1.93679 |
|
Na |
-2889.17 |
1238.549 |
373.4366 |
-1.75428 |
|
Ni |
-10.6024 |
313.1411 |
94.41561 |
-6.93862 |
|
V |
-15.956 |
355.4829 |
107.1821 |
-6.11216 |
|
Zn |
-65.8836 |
416.6489 |
125.6244 |
-5.21486 |
|
|
d̅=-655.11 |
|
||
As presented in Table 10, and according to the tabulated values for the two-sided t-distribution, it is observed that for t0.05 (t=1.8) and t0.025 (t=2.2), all elements exhibit significant differences, except for Ca, Cr, Cu, Fe, K, and Na. This implies that the two methods do not agree with each other, except in the case of Ca, Cr, Cu, Fe, K, and Na.
Turek et al. [17], conducted an investigation on various digestion procedures, namely drying and microwave digestion, ignition and microwave digestion, and drying and conventional digestion, for the purpose of evaluating heavy metal content in sludge samples. The results obtained indicate that the most effective method was ignition and microwave digestion.
Microwave-assisted and ultrasonic-assisted digestion techniques were applied prior to analysis using inductively coupled plasma optical emission spectroscopy, as described by Naicker etal. [8]. The authors concluded that both digestion methods yielded similar levels of accuracy, suggesting their suitability for precise determination of the target metals. Dlamini et al. [7] validated the microwave assisted digestion method for determining heavy metal concentrations in soil from sludge land, using ICP/MS. Their conclusion indicates that all validated parameters fell within acceptable limits, indicating the suitability of the method for its intended purpose. Abegunde et al. [18] conducted a study comparing three conventional acid digestion procedures for soil samples. Their findings revealed that the behavior of each metal towards the digestion acid can guide the selection of the appropriate digestion procedure. In an inter-laboratory study carried out by Santoro et al. [19], different digestion methods were employed for a sewage sludge certified reference material. The results obtained demonstrated no significant differences between the extraction methods used. Two digestion techniques were compared and applied to real soil samples and standard reference materials for the analysis of Sb using ICP/MS. The recoveries of Sb achieved through HF in the acid digestion mixture in a closed-vessel microwave digestion system were found to be excellent, and the concentrations obtained were in very good agreement with certified or reported concentrations of reference materials. Monlau et al. [21] focused their study on anaerobic digestion with pyrolysis in soil. The results obtained indicated that both solid-digestate and pyrochar exhibited favorable properties as soil amendments, albeit with complementary effects.
Conclusion
The comparison of dry digestion method and wet digestion method show that the microwave digestion method was superior for analyzing all elements in soil samples, except for calcium (Ca), chromium (Cr), and magnesium (Mg). On the other hand, the dry digestion method is recommended for the analysis of calcium (Ca), copper (Cu), chromium (Cr), magnesium (Mg), sodium (Na), nickel (Ni), and vanadium (V) base on the present study. Based on the obtained results, the conclusion is drawn that microwave digestion is suitable for sample preparation for various soil types, including soil, sandy soil, and clay samples.The microwave digestion method is recommended for use in determining heavy metal concentrations in soil and river basin samples. This recommendation suggests that the microwave digestion method is reliable and effective for preparing samples and extraction of heavy metal content in environmental samples.
Acknowledgment
The author acknowledge the Deanship of Scientific Research at King Faisal University, for the financial support under Ambitious Researcher (Grant No.0000).
Conflicts of Interest
The author declare(s) that there is no conflict of interest regarding the publication of this paper.
Funding Sources
There are no funding sources.
References
- Da Silva, Y.J.A.B., do Nascimento, C.W.A. & Biondi, C.M. Comparison of USEPA, digestion methods to heavy metals in soil samples. Environ Monit Assess, 2014 186, 47–53 https://doi.org/10.1007/s10661-013-3354-5
CrossRef - Chen, M., Ma, L. Q., & Harris, W. Comparison of four USEPA digestion methods for trace metal analysis using certified and Florida soils. Journal of Environmental Quality,1989 27, 1294–1300
CrossRef - Kántor, T. Electrothermal vaporization and laser ablation sample introduction for flame and plasma spectrometric analysis of solid and solution samples. Spectrochimica Acta Part B: Atomic Spectroscopy, 2001, 56, 1523–1563.
CrossRef - Axelsson, M., &Rodushkin, I. Determination of major and trace elements in sphalerite using laser ablation double focusing sector field ICP-MS. Journal of Geochemical Exploration, 2001, 72, 81–89.
CrossRef - Belarra, M. A., Resano, M., Vanhaecke, F., & Moens, L. Direct solid sampling with electrothermal vaporization/atomization: what for and how? TrAC Trends in Analytical Chemistry, 2002, 21, 828–839.
CrossRef - Al-Harahsheh, M., Kingman, S., Somerield, C., & Ababneh, F. Microwave-assisted total digestion of sulphide ores for multi-element analysis. Analytica Chimica Acta,2009, 638, 101–105.
CrossRef - Hlengiwe Dlamini, Precious Mahlambi& Sihle Mngadi Validation of microwave-assisted digestion and Inductive coupled plasma -mass spectrometer for the determination of trace metals in the soil around Darvillsludgeland and their environmental complications, Soil and Sediment Contamination: 2023,32:6, 637-651, DOI: 10.1080/15320383.2022.2123446
CrossRef - Kavisha Naicker, Precious Mahlambi&MphilisiMahlambi Comparison of ultrasonic and microwave assisted digestion methods for the determination of heavy metals in soil and sediment: The effect of seasonal variations on metal concentrations and risk assessment, Soil and Sediment Contamination,2023, 32:3, 320-336, DOI: 10.1080/15320383.2022.2084032
CrossRef - Operacz, Agnieszka, Adrianna Bigaj, Karolina Hap, and Tomasz Kotowski. “The Effect of Sample Preparation and Measurement Techniques on Heavy Metals Concentrations in Soil: Case Study from Kraków, Poland, Europe” Applied Sciences,2022,12:4,2137-2141. https://doi.org/10.3390/app12042137.
CrossRef - Isabel Greenberg, Anja Sawallisch, Jan Stelling, Michael Vohland, Bernard Ludwig, Optimization of sample preparation and data evaluation techniques for X‐ray fluorescence prediction of soil texture, pH, and cation exchange capacity of loess soils, Soil Science Society of America Journal,2023,1-16 https://doi.org/10.1002/saj2.20598.
CrossRef - Chattopadhyay, P., Fisher, A., Henon, D. et al. Matrix Digestion of Soil and Sediment Samples for Extraction of Lead, Cadmium and Antimony and Their Direct Determination by Inductively Coupled Plasma-Mass Spectrometry and Atomic Emission Spectrometry. Microchim. Acta,2004,144, 277–283. https://doi.org/10.1007/s00604-003-0113-2.
CrossRef - Ahmed, A.Y., Abdullah, M.P. &Siddeeg, S.M.Environmental hazard assessment of metals in marine sediments of Sabah and Sarawak, Malaysia. Int. J. Environ. Sci. Technol, 2023,20, 7877–7886. https://doi.org/10.1007/s13762-022-04514-z.
CrossRef - Mingtao Xiang, Yan Li, Jiayu Yang, Kaige Lei, Yi Li, Feng Li, Daofu Zheng, Xiaoqian Fang, Yu Cao, Heavy metal contamination risk assessment and correlation analysis of heavy metal contents in soil and crops, Environmental Pollution,2021,278,1169-1180. https://doi.org/10.1016/j.envpol.2021.116911,
CrossRef - Xi Zhong, Ziwu Chen, Yaying Li, Kengbo Ding, Wenshen Liu, Ye Liu, Yongqiang Yuan, Miaoyue Zhang, Alan J M Baker, Wenjun Yang, Yingheng Fei, Yujie Wang, Yuanqing Chao, Rongliang Qiu, Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China, Journal of Hazardous Materials, 2020,400, 22-32. https:// 10.1016/j.jhazmat.2020.123289
CrossRef - Soylak, M.; Tuzen, M.; Narin, I.; and Sari, H. Comparison of microwave, dry and wet digestion procedures for the determination of trace metal contents in spice samples produced in Turkey,” Journal of Food and Drug Analysis:2004,12:3,254-258
https://doi.org/10.38212/2224-6614.2634
CrossRef - Shigeki Tsuneya, Makoto Nakajima, Yohsuke Makino, Suguru Torimitsu, Rutsuko Yamaguchi, Hirotaro Iwase, A quantitative comparison between using sodium hypochlorite as a digestion method for the diatom test and the conventional method using fuming nitric acid, Forensic Science International,2021,329, 86-111. https://doi.org/10.1016/j.forsciint.2021.111086
CrossRef - Turek, Anna, Kinga Wieczorek, and Wojciech M. Wolf., Digestion Procedure and Determination of Heavy Metals in Sewage Sludge—An Analytical Problem” Sustainability,2019,11, 6: 17-53. https://doi.org/10.3390/su11061753
CrossRef - Abegunde S. M., Oyebanji A. O., OsibanjoO.m., Evaluation of Digestion Procedures on Heavy Metals in Soil of a Dumpsite in Ibadan, South-western Nigeria, Suan Sunandha Scienceand Technology Journal,2018,5, 2, 1-5 10.14456/ssstj.2018.6
- Anna Santoro, Andrea Held, Thomas P.J. Linsinger, Andres Perez, Marina Ricci. Comparison of total and aqua regia extractability of heavy metals in sewage sludge: The case study of a certified reference material, TrAC Trends in Analytical Chemistry, 2017,89, 34-40, https://doi.org/10.1016/j.trac.2017.01.010.
CrossRef - Amereih, S., Meisel, T. ., &Wegsheider, W., Accurate Determination of Total Antimony Using ICP-MS and Optimization Its Extraction Efficiency From Reference and Soil Samples. Palestine Technical University Research Journal, 2018,6:1, 48–58. https://doi.org/10.53671/pturj.v6i1.56.
CrossRef - Monlau F., Francavilla M., Sambusiti C., Antoniou N., Solhy A., Libutti A., Zabaniotou A., Barakat A., Monteleone M., Toward a functional integration of anaerobic digestion and pyrolysis for sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment, Applied Energy,2016, 169, 652-662. https://doi.org/10.1016/j.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










