Determination of Caffeic Acid in Cigarette Smoke and Urine by Electrochemical Methods Using Supramolecular Electroactive Materials grafted in Screen Printed Carbon Electrode
Syed Kashif Ali1* , Geetu Gambhir2
, Geetu Gambhir2 , Gulrana Khuwaja3
, Gulrana Khuwaja3 , Sayeed Shabi4, Safaa F. Saleh4,5
, Sayeed Shabi4, Safaa F. Saleh4,5 , Angum M. M. Ibrahim5 Amal. A. Noureideen6 Mawada Abubaker Abdelgadir Mohammed7 and Sumathi Nagarajan4
, Angum M. M. Ibrahim5 Amal. A. Noureideen6 Mawada Abubaker Abdelgadir Mohammed7 and Sumathi Nagarajan4
1Department of Chemistry, Faculty of Science, Jazan University, Jazan, Saudi Arabia.
2Department of Chemistry, Acharya Narendra Dev College, University of Delhi, Govindpuri, Kalkaji, New Delhi, India.
3Department of Nursing, Farasan University College, Jazan University, Jazan, Saudi Arabia.
4Department of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Fayoum University, Fayoum, 63514, Egypt.
5Al-Rayan Private College for Health Science and Nursing, Al Madinah Al Munawarah, Saudi Arabia.
6Department of Clinical Chemistry, College of Pharmacy, Jazan University, Jazan, Saudi Arabia.
Corresponding Author E-mail: skali@jazanu.edu.sa
DOI : http://dx.doi.org/10.13005/ojc/390606
Article Received on : 07 Nov 2023
Article Accepted on : 08 Dec 2023
Article Published : 21 Dec 2023
Reviewed by: Dr. Sachin Kamble
Second Review by: Dr. Himanshu Agarwal
Final Approval by: Dr. S. A Iqbal
Selective determination of caffeic acid, was carried out by using Screen Printed Carbon Electrode (SPCE) to give a reliable, scalable, and inexpensive electrochemical method with enhanced analytical performance. SPCE was first activated by linear sweep voltammetry using KOH solution, followed by its electrochemical grafting with cyclodextrins. Britton Robinson buffer (BRB) solution having pH 8.1, demonstrated a clear electrocatalytic impact towards caffeic acid oxidation, which indicated a greater current response compared to the activated electrode in the cyclic voltammetric and square wave voltammetric (SWV) investigations. The SWV waves at the activated SPCE was used under ideal conditions to produce a linear calibration curve with detection limits of (LoD, 4 sb/m) and quantification limits of (LoQ, 11 sb/m) of 0.38 M and 1.1 M, respectively.
KEYWORDS:Cigarette Smoke; Determination of Caffeic acid; Electrochemical methods; Materials
Download this article as:| Copy the following to cite this article: Ali S. K, Gambhir G, Khuwaja G, Shabi S, Saleh S. F, Ibrahim A. M. M, Noureideen A. A, Mohammed M. A. A, Nagarajan S. Determination of Caffeic Acid in Cigarette Smoke and Urine by Electrochemical Methods Using Supramolecular Electroactive Materials grafted in Screen Printed Carbon Electrode. Orient J Chem 2023;39(6). |
| Copy the following to cite this URL: Ali S. K, Gambhir G, Khuwaja G, Shabi S, Saleh S. F, Ibrahim A. M. M, Noureideen A. A, Mohammed M. A. A, Nagarajan S. Determination of Caffeic Acid in Cigarette Smoke and Urine by Electrochemical Methods Using Supramolecular Electroactive Materials grafted in Screen Printed Carbon Electrode. Orient J Chem 2023;39(6). Available from: https://bit.ly/4axGDqs |
Introduction
Caffeic acid (CFA) is ubiquitous because it is a biosynthetic intermediate for lignin, a major component of woody plant material and its byproducts 1. Due to ganglionic blockade, high doses of caffeic acid induce bradycardia, hypotension and depressed mental status 2. However, there are findings such as excessive use of electronic cigarettes lead to potentially fatal doses of it 3 leading to the harmful impacts of orally ingested caffeic acid. However, caffeic acid has also been shown to have medicinal applications in the treatment of cardiovascular disease, respiratory disorders, lung cancer, and age-related neurological disorders like Alzheimer’s and Parkinson’s disease 4. Tobacco use increases the risk of negative effect towards COVID-19, accelerates the virus’s development, and increases the risk of death, as studies have shown 5.
Tobacco users are less likely to be hospitalised with COVID-19 according to some findings 5. However analysis of data from multiple studies disputes the claim of smoking being linked to severity of coronavirus illness. Caffeic acid analysis is therefore essential in both the cigarette manufacturing facility and forensic toxicology 6. Many different analytical methods like HPLC 7,8, mass spectrometry9-11, spectrophotometry 12, capillary electrophoresis 13, high-performance capillary electrophoresis 14, capillary electrophoresis coupled with electrochemiluminescence 15, and sensors 16 have been used to determine caffeic acid. However, these procedures are extremely costly as they call for cutting-edge technological expertise and skilled labour.
The above mentioned techniques also have the significant flaw of squandering the analyte during sampling and analyzing. The proposed electrochemical methods are preferable because they are cheap, quick and easy to perform.. Caffeic acid is an analyte that oxidizes at greater potential, and as a result presents a challenge when attempting electrochemical detection. Henceforth its measurement necessitates the use of an electrode that can reduce the over-potential by means of a more powerful current. A novel, a screen-printed carbon anode (SPCE) grafted with b-cyclodextrin was prepared and experimented to accomplish this.
Electrochemical grafting with benzene diazonium salts yields stable and reactive aryl radicals that bond firmly to substrate surfaces 17,18 by covalent bonds By reducing aryl diazonium cations and oxidising the secondary hydroxyl groups of b-cyclodextrin, organic moieties can be attached to active sites on the carbon surfaces via carbon and nitrogen linkages, resulting in a covalent connection 19.
Experimental
Material and Methods
The SPCE and the chemicals used for the experimental work viz. caffeine (high grade quality), uric acid, b-cyclodextrin, acetic acid, NaNO2, HCl, boric acid, glucose and phosphoric acid were purchased from Merck USA. Deionized water was used to make solution of Caffeic acid, while all other compounds/solvents of a normal laboratory grade were used as such. Caffeic acid concentrations were determined using a Britton Robinson buffer solution.
The CHI760D electrochemical work station CH Instruments, USA) was used for the electrochemical experiments. The JEOL JSM-7401F FESEM was used to examine the surface images of electrodes. The active electrode, reference electrode( Ag/AgCl pellet ) and the auxiliary carbon electrode were all coated with SPCE using a drop coating process. Caffeic acid was measured using SWV at a frequency of 1.1 kHz and a potential range of 0.5 to 1.5 V. The optimal pulse amplitude was 60 mV and the step potential was 10 mV.
General procedures
Grafting of SPCE
In-situ generated b-cyclodextrin ( β -CB) was used to modify the electrode surface through electrochemical grafting. The diazonium cation was synthesized by addition of saturated solution of NaNO2 to 5 mM β -CB solution (kept at 0-50C) in 0.5 M HCl at room temperature, which resulted in a final concentration of 10 mM. The reaction mixture was cooled in an ice bath for 5 min. Thereafter response of SPCE was observed for 0.0 to -1.0 V by linear sweep voltammetry for 15 cycles at 100 mV s-1 to transplant it. After the modifications, the new assembly electrode was thoroughly cleaned with streaming distilled water and was dried and flushed with nitrogen gas before being put to use.
Electrochemical measurements
The square wave voltammetry was used to conduct the electrochemical experiments on β -CB grafted SPCE. Caffeic acid was measured by dropping a standard test solution of the compound into a Britton-Robinson buffer solution at pH 8 and allowing it to fill the entire electrode for 25 sec at a potential of -0.55 V before cycling to +1.5 V.
Preparation of Urine sample
A healthy adult volunteer’s urine was collected and stored in sterile plastic containers. After 5 minutes of stirring with a magnetic stirrer, the urine was filtered through Whatman filter paper to make a clear filtrate. Then, 5 mL of the filtrate was poured into a 50 mL volumetric flask, and the flask was filled to the standard mark with a BR buffer solution having the standard pH of 8. The recovery analysis was examined by adding standards of varying caffeic acid amounts to the filtrate and analyzing the mixture with SW Voltametery.
Caffeic Acid Extraction from Cigarette
Extracts from cigarettes to be analyzed were prepared by following the method given and detailed 21. The sample of tobacco was obtained by peeling the rolling paper and placing the tobacco in a spotless watch glass. The sample of tobacco was then dried in a vacuum furnace for 3 h at 35 ºC. Dry tobacco (0.25 g) was crushed and added to 30 mL of distilled water. The mixture was sonicated for 5 h in the ultra sonicator bath to isolate caffeine. The insoluble brown residue was filtered, and the water soluble caffeine was poured into 100 mL standard flask, and diluted to the appropriate concentrations as recquired. It was then stored at 4 ºC for further use in experiments.
Results and Discussion
Characterization and surface morphology of SPCE was caried out by using the analytical techniques of FT-IR and a FESEM for, different time phases of application. Fig.1 shows the FT-IR measurements made on SPCE with naked electrodes, activated electrodes and electrodes grafted with β -CB. Both activated SPCE and SPCE with β -CB grafted showed broad OH stretching extending between 3500 and 3320 cm-1 22. Due to the existence of an additional hydroxyl (-OH) group in β -CB, the broadening of the -OH stretching band in the grafted SPCE is observed as compared to the activated SPCE. An evidence for the grafting of β -CB on the surface of the electrode is indicated by the presence of a hydroxyl stretching observed at 1380 cm-1 and an aromatic stretching at 1650 cm-1 23. The UV-Vis spectroscopic analysis (Fig. 2) verified the covalent attachment of -CB, with a spectral band at 250 nm indicating the presence of a benzene ring.
 |
Figure 1: FTIR spectrums of bare (a), activated (b), and β-CB grafted SPCE (c) |
 |
Figure 2: UV spectrum of bare SPCE (a) and β-CB grafted SPCE (b). |
A resolved image of untreated SPCE (Figure 3A) reveals a dense coating of graphite particles on the carbon surface; a similar image of activated SPCE (Figure 3B) reveals morphological alterations on the surface of untreated SPCE as a result of activation. While the untreated SPCE surface is smoth and uniform, the carbon surface deteriorates upon electrochemical activation. After activation, the SPCE surface became rough and porous. Since the oxidised carbon surface was exposed, after the carbon was dissolved by the basic media, the surface was likely to be turning rough and permeable. Fig. 3C is an example of a high-resolution scanning electron micrograph demonstrating the widespread distribution of β -CB affixed to SPCE.
 |
Figure 3: Surface morphology of three different samples was examined: untreated SPCE (sample A), activated-SPCE (sample B), and β-CB grafted SPCE (sample C). |
Electrochemical Characterization of β -CB Modified Electrode:
The performance of electrodes was monitored using the cyclic voltammetry with a ferri/ferrocyanide couple. This instrument was used to verify electrode surface modifications and to probe electron transmission or blocking behavior. The results illustrated in Fig. 4, shows the characteristic redox peak of ferri/ferrocyanide in the two samples of bare and activated SPCE, but a diminished redox peak shape and very high peak separation in the β -CB grafted SPCE. This result demonstrates that the β -CB forms strong covalent bonds with the cathode surface. The negatively charged terminal hydroxyl acid group is to blame for the shift, as it prevents probe passage towards the electrode surface.
 |
Figure 4: A. Nyquist plots of 5 mM [Fe(CN)6]4–/3– at; (a) bare SPCE (b) β-CB/SPCE B. Nyquist plots of 5 mM [Fe(CN)6]4–/3– at (a) pH 3.5, (b) pH 5.5 and (c) pH 7 |
Electrochemical Behavior of CFA at β -CB grafted SPCE
Electrochemical characterization of bare, activated and β -CB grafted SPCE towards 100 mM caffeic acid by linear and square wave voltage sweep in BRB solution (pH = 8.1) was carried out. The oxidation peaks of voltammograms are depicted in Fig. 5, which clearly exhibited the irreversible manner. There was no discernible oxidation peak at the unmodified SPCE, but a tiny peak current was observed at the activated SPCE. However, at a positive potential of +0.899 V, the current through the β -CB grafted SPCE was observed to be higher. This findings distinguish β -CB grafted SPCE from activated SPCE in terms of its electrocatalytic activity towards caffeic acid (Fig. 6).
 |
Figure 5: (I) (a) Caffeic acid concentration (CV) in BRB solution pH 8.1: 0 μM (b), 100 mM (c), b– B grafted SPCE (II) (a). Caffeic acid SWV in BRB solution pH 8.1 showed 100 mM in activated SPCE and (b) b -CB grafted SPCE. |
 |
Figure 6: The linear sweep voltammogram (LSV) obtained for the diazotation mixture, consisting of 10 mM NaNO2 and 5 mM β-CB in a 0.5 M HCl solution, on active screen-printed carbon electrodes (SPCE). |
Effect of scan rate
Determination of control of electrochemical process at β -CB grafted SPCE by diffusion or adsorption was carried out through scan rate studies. The cyclic voltammetry experiments were performed at the concentration of 100 mM caffeic acid in BRB solution (pH 8.1) with scan rates ranging from 50 mV s-1 to 200 mV s-1 for determination of the effect of scan rate on peak potentials and the peak currents. Oxidation was confirmed to be irreversible after a peak was detected during the anodic scan from 0.25 to 1.45 V at all scan rates with positive shifts on peak potential (Fig. 7). Diffusion-controlled process at the electrode, was indicated by a straight line with a slope of 0.365 and plot of the log of peak current against the log of scan rate. gave the a regression coefficient of 0.998 .
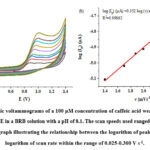 |
Figure 7: The cyclic voltammograms of a 100 μM concentration of caffeic acid were obtained using a β-CB grafted SPCE in a BRB solution with a pH of 8.1. |
Effect of pH
By changing the pH from 5.0 to 10.0, we were able to assess the role of pH in the determination of caffeic acid on β -CB grafted SPCE and found a positive peak potentials shift with rising pHs. At the same time, the peak current rises from 5.0 to 10.0 and falls above pH = 8.1. The monoprotonated and diprotonated forms of the diacidic weak basic caffeic acid have pKa values of 8.22 and 3.22, respectively (Fig. 8). Caffeic acid, in its un-protonated and alkaline state, has an excess of the supporting electrolytes at a pH of 9.0 or lower. The monoprotonated form of caffeic acid is formed when the caffeinate is protonated at a pH of 7.2–8.5, while the diprotonated form is the predominant one at a pH of 2.5–2.8 [24,25]. The linear regression equation, E (V) = -0.0638pH + 1.332 (R2 = 0.9965), describes the connection between the peak potential and the pH. If the slope is close to 0.0645, then the number of electrons and protons in the anode are similar 26.
 |
Figure 8: (a) The cyclic voltammograms (CVs) of 100 μM caffeic acid solution in a buffered aqueous solution (BRB) were obtained by altering the pH values within the range of 5.0 to 9.5. |
Optimization of SWV parameters
The SWV method was used to determine caffeic acid at the changed electrode because it provided a stronger signal and was more sensitive. A distinct oxidation peak was seen for caffeic acid at a concentration of 100 μM in BRB solution with a pH of 8.1. Through manipulation of the frequency, amplitude, and step potential of the SW parameters, we conducted an investigation into the effects on the current reaction. An increase of the requirements resulted in an elevated magnitude of the peak current that was observed. The anodic peak form and peak current magnitude determined that a step potential of 10 mV, amplitude of 60 mV, and frequency of 40 Hz delivered the best results.
Effect of Accumulation Potential and Time
Between -0.5 and +0.5 V, the impact of accumulation potential on the reaction to 100 mM caffeic acid was examined. When the accumulation potential was changed over the course of 15 seconds, the peak current rose linearly with the potential, from a low of -0.5 V to a high of 0.1 V. Higher than 0.1 V, the current began to drop. As a result, 0.1 V was selected as the optimal buildup potential. Similar research was conducted on the impact of accumulation time between 25 and 65 s using an accumulation potential of 0.1 V, with the maximum value being obtained at 35 s.
Square wave Voltammetric Studies for Caffeic Acid Determination
Caffeic acid was determined using square wave voltammetry with the optimized measurable variables. The concentration-dependent SW voltammograms of β -CB grafted SPCE in BRB solution (pH 8.1) with different concentrations of caffeic acid are displayed in Fig. 9. The highest current versus concentration curve followed a linear relationship between 0.5 and 300 mM. Limit of detection was determined to be 0.35 mM based on the linear relationship, with the regression equation Ip (mA) = 1.778 (mM) + 5.296 and R2 = 0.998.
 |
Figure 9: (a) Effect of square wave voltammetric (SWVs) at different concentration of caffeic acid (0.5, 10, 25, 50, 100, 150, 200, 260 and 300 mM in BRB solution pH 8.1); |
Repeatability, reproducibility and stability of the modified electrodes
The SWV signals for 100 mM caffeic acid in BRB solution (pH 8.1) were measured to examine the repeatability of β -CB attached SPCE. After taking ten observations in a row, the peak currents at the same electrode had a relative standard deviation of 2.56%. Instead, three SPCE electrodes were made using the same protocol and their responses were compared to determine whether or not the β -CB grafted SPCE could be reliably reproduced. Current peaks recorded at three different electrodes had a relative standard deviation (RSD) of 2.50%. After 10 days of being left open to the air, the stability of a b -CB grafted SPCE was tested and found that it maintained 96.84% of its initial outcome. These results demonstrate the β -CB grafted SPCE’s high repeatability, reproducibility and durability against caffeic acid oxidation.
Interference study
The SWV signals for 100 mM caffeic acid in BRB solution (pH 8.1) ,the potential contamination in the detection of caffeic acid using β -CB grafted SPCE. In 50-fold concentrations of caffeic acid, current reactions were unaffected by Fe3+, Mg2+, Ca2+, K+, Na+, NH4+, uric acid or glucose (Table-1). As a result, the presence of the potential interfering species does not prevent the modified electrode from being used for caffeic acid detection, suggesting that these species do not substantially interfere with the determination of caffeic acid. The measurement of caffeic acid is unaffected by the presence of the interfering substances, which are found in both human urine and cigarette samples.
Table 1: Effect of Some Interfering Foreign Ions on the Peak Current Response of 100 mM Caffeic Acid at β -CB/SPCE.
|
Interferents |
Concentration of Interferents (mM) |
Signal Change |
|
uric acid |
5000 |
3.01 |
|
D-glucose |
5000 |
1.95 |
|
Fe3+ |
5000 |
1.91 |
|
Ca2+ |
5000 |
2.36 |
|
Mg2+ |
5000 |
2.41 |
|
K+ |
5000 |
0.45 |
|
Na+ |
5000 |
0.23 |
|
NH4+ |
5000 |
0.09 |
Analytical Applications
Human urine samples and cigarette samples were analyzed to determine how useful β -CB grafted SPCE would be in real life situations. Cigarette and pee samples were diluted with BRB solution pH 8.1 before applied. The samples were recovered by mixing in the appropriate amount of caffeic acid solution. Table-2 depicts the findings of cigarette and urine sample recoveries, respectively. The recovery data demonstrates that caffeic acid was accurately detected in cigarette and urine samples.
Table 2: Determination of Caffeic Acid Levels Urine and Cigarette Samples using β -Cb Grafted Spce (n=3).
|
Sample |
Added (μM) |
Found (μM) |
Recovery (%) |
|
Urine |
5 |
4.88 |
97.60 |
|
10 |
10.38 |
103.80 |
|
|
25 |
25.56 |
102.24 |
|
|
Cigarette (Gold Flake, ITC Ltd.) |
50 |
50.8 |
101.6 |
|
100 |
105 |
105.0 |
Conclusion
In summary, using electrochemical techniques, b-cyclodextrin ( β -CB) was grafted onto the surface of SPCE. The grafting of β -CB onto the SPCE surface is supported by the FTIR and VU-VIS spectra, which revealed the existence of aromatic and hydroxyl functional groups. The electrocatalytic performance of β -CB grafted SPCE on caffeic acid is better, despite its lower conductivity compared to that of bare SPCE. Caffeic acid was detected over a linear range of 0.5–300 mM by β -CB grafted SPCE under optimum circumstances, having a low detection limit. Additionally, caffeic acid content in cigarette products and urine samples within the range has been effectively identified using the prepared electrode.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Ma ,Q.H. and Xu ,Y.P. ; Biochimie, 2008, 90, 515
CrossRef - Silva, H. and Lopes,N.M.F. ; Front. Physiol., 20201, 595516
- Akyol,S., Ugurcu,V. Altuntas, Hasgul, Cakmak ,A. R. O. and Akyol, O. ; The Scientific World J., 2014, 561971
CrossRef - Shwetha,B. Sudhanva,M.S. Thimmegowda,N.R. Anil Kumar, B.M. Jagadeesha, Guddappa,G.S. Prasanna,H. D.S. Dinesh, Shobith ,R. R. and Anandakumar,C.S. Asian J. Chem.,2022 34, 2183
CrossRef - Gupta,A.K. Nethan ,S.T. and Mehrotra,R. Respir. Med. ;2021176, 106233
- Gaillard ,Y. and. Pépin, GJ. Chromatog. A, ; 1997, 763, 149
CrossRef - Ye, J.-C. Hsiao,M.-W. Hsieh, C.-H. W Wu,.-C. Hung, Y.-C. and Chang, W.-C. Taiwan. J. Obstet. Gynecol. ; 2010,49, 266
CrossRef - Luo,X.-B. Chen,B. Yao, S.-Z. and Zeng, J.-G. J. Chromatogr. A, ; 2003, 73, 986
CrossRef - Razboršek, M.I. Ivanović ,M. and Kolar, M. Molecules, ; 2021,26,2475
CrossRef - Liu,F. Li, L. Cai ,W. and Shao,X. ;Chromatographia,2009 69, 743
CrossRef - Wu,Z.-J. Ma,X.-L. Fang,D.-M. Qi, H.-Y. Ren, W.-J. and Zhang, G.-L. ; Eur. J. Mass Spectromet., 2009,15, 415
CrossRef - Cai, Z. Yang, Yu ,F. F. and Lou, Nan Fang Yi Ke Da Xue Xue Bao, L.-J. 29, ; 2009 1907 (in Chinese).
- Zhang,Y. Kong, C. and Liu,Y. , ; Asian J. Chem.,2015 27, 2154
CrossRef - Zhang, L.L. Dong,S.Q. Yang, Z.Y. Wang,Q.J. He,P.G. Fang,Y.Z. J. Pharm. Biomed. Anal., ; 2008 48, 198
CrossRef - Belay,A. Int. J. Biophys., Belay,2012 2, 12
CrossRef - Pigani, L. C. Rioli, Zanfrognini, B. García-Guzmán, J.J. Palacios-Santander, J.M. and Cubillana-Aguilera, , L.M. Sensors, ; 2022 22,8848
CrossRef - Floch,F.L. Simonato ,J.-P. and Bidan,G. Electrochim. Acta, ; 2009 54, 3075
CrossRef - Hetemi, D. V. Noël and Pinson, J. Biosensors, ; 202010, 4
- Yang,L. Wang,Y. Xu, Zhu,X. Zhang, J. He, R. P. and Fang, Y. Anal. Chim. Acta, ;2011 689, 39
CrossRef - Myles,A. Behan,J.A. Twamley,B. Colavita ,P.E. and Scanlan, E.M. ACS Appl. Bio Mater. ; 20181, 825
- Florescu,A. Ferrence, R. Einarson, Selby,T. P. Soldin ,O. and Koren, G. Ther. Drug Monit., ;2009 31, 14
CrossRef - Hou,X. Zhang,W. He, M. Lu,Y. Lou ,K. and Gao, F. Asian J. Pharm. Sci., ;2017, 12, 558
CrossRef - Danek,M. Korytkowska-Wałach, A. and Barchańska, H. Molecules, ;2021, 26, 5945
CrossRef - Genaro-Mattos, T.C. Maurício, Â.Q. Rettori, Alonso ,D. A. and Hermes-Lima, M. PLoS ONE, ;2015,10, e0129963
CrossRef - Arciszewska,Z. Gama, Kalinowska Swiderski,,S. Swisłocka,M. Golebiewska, G. R. E. Naumowicz, M. Worobiczuk, M. Cudowski, A. . Pietryczuk, De Stefano, AC. Milea, Lewandowski ,D. W. and Godlewska-Zyłkiewicz, B. Int. J. Mol. Sci., Godlewska-Zyłkiewicz, ; 2022,23, 888
CrossRef - Trnková,L. Friml, J. and Dračka, O. Bioelectrochemistry, ;2001,54, 131
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










