Synthesis and Characterization of 6-Amino Caproic Acid Tkp-Based Resin for Wastewater Treatment
Department of Chemistry J.N.V. University, Jodhpur, 342001, Rajasthan, India.
Corresponding Author E-mail: sugansodha@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390536
Article Received on : 07 Aug 2023
Article Accepted on : 20 Sep 2023
Article Published : 05 Oct 2023
Reviewed by: Dr. Rajkumar Raikar
Second Review by: Dr. Divya Rathi
Final Approval by: Dr. Pounraj Thanasekaran
The natural polysaccharide-based resin of tamarind using functional group as 6-amino caproic acid has been synthesized by porath’s method. Their adsorption behavior also evaluated by determination of % adsorption of toxic metal ions from stock solution as well as industrial effluents by batch method and column separation experiment. Their physiochemical properties as bulk density, ion exchange capacity, and FTIR, were investigated. Using the batch equilibrium approach, Kd of five metal ions at various pH levels was investigated. The maximum removal % of metal ions of Cu+2, Fe+2, Zn+2, Cd+2, and Pb+2 at TACA resin were found at 97.97%,97.80%, 96.39%, 94.94%, and 91.90% respectively. Distribution ratio of these ions observed from pH 2 to 8 was Cu+2 > Fe+2> Zn+2> Cd+2 > Pb+2. The toxic metal ions from discharge samples of the metallurgical and mineral industries have been removed using ion exchange resins. Recovery of Zn (Ⅱ), Pb (Ⅱ), Cd (Ⅱ), Cu (Ⅱ), and Fe (Ⅱ) were obtained at 95.44, 96.27, 96.41, 98.82, and 100% respectively. The ion exchange capacity of TACA resin was found 2.24mmol/g
KEYWORDS:Column experiment; Distribution coefficient; Ion exchange capacity; Tamarind 6-amino caproic acid
Download this article as:| Copy the following to cite this article: Kanwar S, Singh A. V. Synthesis and Characterization of 6-Amino Caproic Acid Tkp-Based Resin for Wastewater Treatment. Orient J Chem 2023;39(5). |
| Copy the following to cite this URL: Kanwar S, Singh A. V. Synthesis and Characterization of 6-Amino Caproic Acid Tkp-Based Resin for Wastewater Treatment. Orient J Chem 2023;39(5). Available from: https://bit.ly/3ZKKgUU |
Introduction
Water is the prime resource on land because all living things must survive. One of the basic needs for all life on earth is access to clean drinking water. But there are many factors that contaminant water directly or indirectly. Natural and manmade processes could have contributed to the presence of these heavy metals on the water’s surface. Natural activities include the weathering of metal-containing rocks, volcanic eruptions, forest fires, and naturally occurring weathering processes. As a result of these actions, metal enters the various ecosystems and causes various problems to living beings. When toxic metal ions accumulate by ecosystem, they can cause serious health issues because they enter the food chain and eventually the human body. Such pollutants are acutely hazardous, even in one part per billion1.
Firstly, these metal ions combine with water and oxygen to form hydrous oxide then accumulate and flocculate on the surface of wastewater. It is significantly reduced by improvement methods used by metallic companies themselves. Although the lime treatment significantly lowers the aggregation of toxic metals, the residual metal ion flock is still too high to be released into the stream2. As a result, more advanced tertiary treatment is required to eliminate hazardous metal ions.
These harmful metals can be successfully brought to a safe level by the use of an ion exchanger3. The removal of poisonous and costly metal ions from industrial discharge that contains high levels of alkali and alkaline earth metal ions is another application for ion-selective exchangers4.
Different natural polymers and their derivatives work as efficient adsorbents to remove toxic metallic ions from wastewater generated by companies5 then treated water released into the environment can reduce the possibility of human diseases and environmental risks. Due to their inherent qualities, such as strong adsorptive ability, low cost, renewability, biodegradability, biocompatibility, and simplicity of modification, polysaccharides have been widely used to create adsorption materials in recent years6. Additional functionalization is provided through grafting, blending, or combining with other nano-materials to boost its potency for heavy metal ion adsorption7.
The addition of a second component to the primary polymer chain improves mechanical strength, which is one of the key conditions for the recyclability of adsorbents. Many polysaccharides as guar gum, cellulose, tamarind and many are using now a days for synthesis of modified ion exchanger resin and they showing good adsorption capacity of toxic metal ions from industrial effluents.8
Chemical structure of TKP
Tamarind kernel powder, which is the source of gum, has a composition similar to cereals, with 12.7–15.4% protein, 3-7.5% oil, 7-8.4% crude fibre, 61–72.2% carbs, and 2.45–3.3% ash. Everything was calculated using a dry weight basis. Tamarind kernel powder is a highly branched carbohydrate polymer chemically speaking. TKP is a polymer with a molecular weight of 52350 Daltons on average and is primarily composed of the three sugars glucose, galactose, and xylose in the molar ratio of 3:2:1. A polymer is made up of a spine that resembles cellulose and has xylose and galactoxylose substituents. Xylose residues (1-6 linked) replace about 80% of the glucose residues, which are then partially replaced by p-1- 2 galactose residues.
 |
Figure 1: Chemical structure of TKP |
The object of my research work was to synthesize and characterization of natural polysaccharide tamarind-based resin using most efficient functional group 6-amino caproic acid which modified ion exchanger adsorption capacity of polysaccharide, boost their potential and extracted metal ions from contaminated water and industrial waste water efficiently.
Materials and Methods
Sample of industrial effluent
Table 1: The characteristic features of the discharge water of Solanki Metal and Steel Industry, Jodhpur (India).
|
Appearance: Turbid |
pH=5.9 |
Colour: Dirty Brown |
Total hardness: 903 |
|
Metal Ions |
Concentration (in ppm) |
||
|
Fe (Ⅱ) Cu (Ⅱ) Zn (Ⅱ) Pb (Ⅱ) Cd (Ⅱ) Cr (Ⅱ) Ni (Ⅱ) Co (Ⅱ) Mg(Ⅱ) Ca (Ⅱ) |
1.87 1.16 3.24 0.79 0.18 0.76 0.74 0.68 113.20 172.00 |
||
Fluoride =0.41; Sulphate = 218.14; Cyanide = 0.02
Synthesis of Tamarind 6- Amino Caproic Acid Resin
The Tamarind 6-amino caproic acid resin was synthesized as following
Preparation of epoxy propyl ether of 6-amino caproic acid
Firstly, 6-amino caproic acid react with epichlorohydrin resulting in the synthesis of compound (Ⅲ) 6-(3-chloro, 2-hydroxy propyl amino) hexanoic acid, which then reacts with NaOH to make (Ⅳ) 6-(2, 3-epoxy propyl amino) hexanoic acid.
An amount of 9.25g (0.1mol) epichlorohydrin was taken in a boiling flask having an attached reflux capacitor. After that 13.11g of a water-based solution of 6-amino caproic acid was added gradually and this mixture was stirred for 3 to 4 hours. At the same time, as the mixture is brought on a magnetic stirrer, 4g (0.1mol) sodium hydroxide dissolved in water was added, and then it was allowed to rotate continuously for 4 hours at Temp 333K.
Preparation of tamarind 6-amino caproic acid (TACA) resin
In the second step 83g of TKP (or 0.5 mol) was dissolved in dioxane and to make it alkaline, caustic soda 50% W/W solution was added. With continual stirring, compound (Ⅳ) solution was added to the alkaline TKP solution, and then it was again stirred for 5 hours at 338 K and left overnight.
In the third step, after retaining it for one night this resin was cleaned using filter paper with aqueous methanol. The yield of resin was 98.2g.
The reaction scheme for synthesis of TACA resin depicts in fig no.2
 |
Figure 2: reaction scheme of synthesis of TACA resin |
Determination of distribution coefficient
The batch approach was used to measure the Kd of metal ions with resin. In every instance, 50 ml of specimen solution was used for the Kd determination, and the pH was regulated using ammonium salts (hydroxide and chloride). The solution’s components were equilibrated by adding 50 milligrams of 6-amino caproic acid TKP resin or stirring the mixture for two hours on a magnetic stirrer. Whatman filter paper no. 40 was used to clear away the solution. Then 4N HCl was used to equilibrate the remaining part on the filter paper and specific Whatman filter paper was used to filter the liquid.9, 10
With the utilization of an AA spectrophotometer, the amount of toxic metal ions in the filtrate and the residue were calculated. By employing AAS to analyze several various reference solutions of metal ions, the calibration curves for various metal ions were plotted. To estimate various metals, the specific resonance line with various wavelengths and oxidizing flame was used. The calibration curves were used to estimate the quantity of metal ion present in the filtrate, and the following formula was used to quantify the percentage of heavy cations removed as well as the distributor coefficient (Kd) on TACA adsorbate.
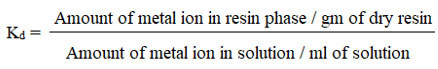
Measurement of removal % of metal ion concentration
AAS spectrophotometer was used to obtain an early metal ion quantity in the solution and filtrate after reaching equilibrium with resin. This formula was employed to calculate the percentage of metal ions removed.
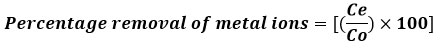
Where Ce constitutes the stable final metal ions concentration in resin and Co represents the initial concentration of metal ions.
Observation
Elimination of metal ions from the wastewater of Solanki metal and steel industry by TACA resin
Table no. 2 displays the findings of TACA resin’s removal % of metal ions from the industrial wastewater of the metal industry. That raising pH causes a first increase, followed by a second drop in the removal % of metal ions, and at pH 7.0 producing the best results. The maximum removal % of ions Cu+2, Fe+2, Zn+2, Cd+2, and Pb+2 found at 97.97%,97.80%, 96.39%, 94.94%, and 91.90% respectively.
Table 2: The removal % of Metal ions from the discharge of steel industries on TACA resin.
|
pH |
The removal % of metal ions |
||||
|
Zn (Ⅱ) |
Cu (Ⅱ) |
Cd (Ⅱ) |
Fe (Ⅱ) |
Pb (Ⅱ) |
|
|
3 |
11.19 |
52.27 |
80.89 |
72.74 |
68.70 |
|
4 |
23.81 |
90.21 |
81.62 |
85.56 |
79.00 |
|
5 |
40.97 |
91.71 |
82.14 |
85.75 |
82.93 |
|
6 |
60.06 |
93.75 |
89.48 |
92.54 |
88.07 |
|
7 |
96.39 |
97.97 |
94.94 |
97.80 |
91.90 |
|
8 |
89.60 |
37.41 |
80.56 |
42.22 |
62.77 |
|
9 |
70.89 |
27.14 |
79.32 |
30.07 |
55.66 |
*maximum % removal of metal ions was found at pH 7
 |
Figure 3: Removal % of toxic metal ions by TACA resin |
Distribution coefficient (Kd) of metal ions in the wastewater of Solanki metal and steel industry
Batch analysis was used to calculate the distributor coefficient (Kd) of the metal ions in the sample solution. The analysis results that with increasing pH, the distribution coefficient values initially increase and later fall. Maximum Kd value was found at 7 pH. Because of the principle of discrimination, the sequence of distribution ratio of these ions observed from pH 2 to 8 wasCu+2 > Fe+2> Zn+2> Cd+2 > Pb+2 which is represented in Fig 3. Distinct metal ions have different distribution coefficients at different pH levels, which points to a potential separation approach for these ions.
At pH 7, the Kd values of the resin yield the best results. The investigation’s findings demonstrate that the resin is highly efficient and has uses in pre-concentration, segregation, and elimination of metal ions (aqueous) produced by the attainment of dissociation equilibrium. The resin is suitable for batch operations on a big scale. By modifying the proportion of interlinking, the pore size of the resin can be regulated, and the resin having suitable interconnecting can be utilized as adsorptive material
Table 3: Distribution coefficient (Kd) of various Metal ions on TACA resin.
|
pH |
Distribution Coefficient of Metal ions on TACA resin (Kd X 103) |
||||
|
|
Zn (Ⅱ) |
Cu (Ⅱ) |
Cd (Ⅱ) |
Fe (Ⅱ) |
Pb (Ⅱ) |
|
3 |
0.73 |
42.20 |
22.65 |
14.85 |
12.26 |
|
4 |
10.99 |
56.15 |
33.99 |
31.41 |
20.51 |
|
5 |
31.55 |
66.42 |
44.19 |
46.87 |
23.55 |
|
6 |
49.38 |
88.16 |
55.56 |
71.35 |
25.74 |
|
7 |
83.46 |
107.76 |
70.15 |
98.28 |
38.12 |
|
8 |
55.25 |
34.07 |
22.56 |
34.81 |
29.94 |
|
9 |
23.88 |
21.54 |
20.98 |
22.89 |
17.36 |
 |
Figure 4: Kd value (distribution coefficient) of metal ions on TACA resin |
Adsorption mechanism of TACA resin
Hydroxyl, carboxyl, and amino functional groups are present in the resin. Whose positions are important in forming complexes with transition metal ions, firstly chelation was done by using -NH2 and -OH group via ionic and ion-dipole interactions11,12,13,14. The ability of resin to adsorb metal ions may therefore be very high, and it may be deduced that complexation with resin takes place by metal ions adsorption.
Column experiment
A glass cylinder having inner width of 1.5 cm and a length of 30 cm was filled with 9.0 gm of TACA resin (9.0g) was utilized in the column experiment. At a volume velocity of 2.0 ml/minute, fifty millilitres of the standard metallic solutions were run over the glass column. A peristaltic pump was used to regulate the flow rate. With 20 ml of fresh water, the column was washed; however, the washing was refused. With varying acid strengths, the metal ions were quantitatively eluted.
Recovery of metal ions
Experiments using column apparatus are useful to analyze the concentration of recovered metal ions. In this method, variable acid concentrations were used to quantitatively elute the metal ion. The Zn+2 was eluted with 0.05 N HCl, Cd+2 with 0.5 N HNO3, Fe+2 was eluted with 0.5 N HCl, Pb+2 with 1N HNO3, Cu+2 was eluted with 2.5 N HCl. After that, deionized water was used to completely wash the resin column. AAS was employed to evaluate the quantities of metal ions in the filtrate solution. Recovery of Zn (Ⅱ), Pb (Ⅱ), Cd (Ⅱ), Cu (Ⅱ), and Fe (Ⅱ) were obtained at 95.44, 96.27, 96.41, 98.82, and 100% respectively.
Table 4: Observational analysis of metal ions recovery on a column of Tamarind 6 – amino caproic acid (TACA) resin.
|
Metal ion |
Amount (mg) |
Amount recovered(mg) |
Recovered metal ion% |
Eluent used |
Volume of eluent (in ml) |
|
Zn(Ⅱ) |
3.12 |
2.98 |
95.44 |
0.05N HCl |
50 |
|
Cu (Ⅱ) |
1.14 |
1.13 |
98.82 |
2.5N HCl |
50 |
|
Cd (Ⅱ) |
0.17 |
0.16 |
96.41 |
0.5N HNO3 |
45 |
|
Fe (Ⅱ) |
1.87 |
1.87 |
100 |
0.5N HCl |
55 |
|
Pb (Ⅱ) |
0.73 |
0.70 |
96.27 |
1.0N HNO3 |
40 |
Ion exchange capacity (IEC) measurement
A back titration procedure was used to determine the resin capacity. 1.0g of resin was placed in an Erlenmeyer flask, followed by 200 ml of standardized NaOH (0.05N) containing 5ml of 5% NaCl solution and allowed to stand overnight. A 25ml volume of supernatant liquid was properly titrated with 0.05N HCl standard stock solution using indicator (phenolphthalein). The equation was used to calculate the newly synthesized resin’s ion exchange ability.

V1= Volume of 0.05N NaOH
V2= Volume of 0.05N HCl
M= Weight of dry resin
Q (mmol/g) = (0.05x 200) – 8(0.05x 19.87) / 0.916
= 2.24
The dry TACA resin’s IEC is observed to be 2.24 mmol/g.
FTIR characterization of TACA resin
The Varian FTIR equipment was used to analyze the TACA resin’s FTIR spectrum with KBr pellets. Polysaccharides are generally appearing in areas 3550-3200 cm-1 for the reason of -OH stretching. The region between 1240-1070 cm-1 possesses a highly intense peak due to the C-O stretching.
A significant spectral band in the range of 3000–2500 cm-1 indicates O–H stretching for the carboxylic functional group. Presence of carbonyl group of carboxylic acid given the fact we get absorption in specific areas. C-H bending is the cause of a second irregular peak at 1600–1500 cm-1.C-N stretching is responsible for a medium peak in the area of 1350-1100 cm-1.
The spectra of resin are given here-
 |
Figure 5: FTIR of TACA resin |
Moisture contents
It is a characteristic property of resin that is affected by variations in cross-linking. The ratio of various monomers used in the polymerization process determines the amount of cross-linking. So, it’s crucial to know how much moisture contains in the modified synthesized resins.
1g of dry TACA resin in ionized form was taken, dried overnight at 800C in a vacuum desiccator to a consistent weight, and weighed. The moisture percentage was found 8.4 %.
Effect of pH
For the complexation of metal ions from water-based solutions, pH is an important factor. Because it influences the amount of the oppositely charged ions on the binding sites of the resin during the reaction and the dissolution of the metal ions. The pH was changed from 2 to 8 to evaluate the relationship between metal ion adsorption % and pH. The pH level affects the adsorption; at certain, metal ion has adsorption rate well, and as pH increases, it becomes less effective. pH 7.0 produced the best results for metal ion adsorption.
Result and discussions
The present research paper was about synthesis and applications of the Tamarind 6-amino caproic acid resin for the elimination of toxic metal ions such as Fe+2, Zn+2, Cu+2, Pb+2, and Cd+2. The resin reduced the amount of harmful metal ions in the water-based solution and industry wastewater by about 94 percent. It was observed that a pH of 7.0 and a stirring duration of 25 minutes were the ideal conditions for the removal of Fe+2, Zn+2, Cu+2, Pb+2, and Cd+2 by TACA resin. The resin is readily found locally in large numbers from farmland production, and as such biopolymers are environmentally friendly, it would be beneficial to employ the TACA resin for the economically efficient treatment of effluent bearing the aforementioned metal ions.
It was discovered that hazardous metal ions can be taken away by the use of imitative TACA resin, with a removal efficiency of almost 94% toxic metal ions that can be taken away from the solution and discharge of Solanki metal and steel industry. Recovery of Zn (Ⅱ), Pb (Ⅱ), Cd (Ⅱ), Cu (Ⅱ), and Fe (Ⅱ) were obtained at 95.44, 96.27, 96.41, 98.82, and 100% respectively.
The experimental data discovered that the pH of the solution and the resin’s molar content concerning the total quantity of metal ions both affected the equilibrium exchange of metal ions. They are used more and more frequently to clean industrial wastewater and drinking water.11-15
Ion exchange capacity was found at 2.24 mmol/g for TACA resin.
Conclusion
These reagents are dual functioning, acting as flocculent cum selective ion binders. They can be used on a large scale on a commercial base. It is cheap and also available in abudance.16
These experimental results which are reported in result section, validated that the TACA resin is an efficient and good adsorbent for deletion of metal ions from industrial effluents rather than others modified polysaccharides-based resin. Because it is eco-friendly natural resin rather than hydrocarbons and poly vinyl-based resin which is expensive and not suitable for ecosystem. So now a day’s demand of natural polysaccharides-based resin is increasing. My suggestion is that we should focus more and more on these types of natural ion exchanger in future.
Acknowledgment
I would like to express thanks to my research guide Dr. Aresh Vikram Singh, Professor, Department of Chemistry, Jai Narain Vyas University, Jodhpur, for his excellent guidance, encouragement, motivation and suggestions. I have no word to express his greatness and kindness.
Conflict of Interest
The authors declare no conflict of interest in the present work
References
- Masindi, V.; Muedi, K. L. Heavy metals., 2018, 10, 115-132.
CrossRef - Mukherjee, A.G.; Wanjari, U.R.; Chakraborty, R.; Renu, K.; Vellingiri, B.; George, A.; Gopalakrishnan, A. V. Journal of Environmental Management., 2021, 297, 113347.
CrossRef - Calmon, C.; Ion exchange pollution control., volume II, CRC Press, 2018.
CrossRef - Zheng, H.; Ding, Y.; Wen, Q.; Liu, B.; & Zhang, S. Resources, Conservation and Recycling., 2021, 167, 105417.
CrossRef - Singh, J.; Kumar, S.; Sharma, S. Biopolymers Springer, Cham., 2022,323-351.
CrossRef - Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Journal of Cleaner Production., 2021, 315.
CrossRef - Sheen Mers, S. V.; Manju, V.; Kamaraj, S. K.; Pérez, M. G. L. Springer., 2022.
- Nayak, A. K.; Pal, D. Functional biopolymers., 2018, 25-56.
CrossRef - Sharma, G.; Pathania, D.; Naushad, M. Ionics., 2015, 21, 1045-1055.
CrossRef - Singh, A. V.; Singh, R. Environmental Progress & Sustainable Energy., 2013, 32(1), 103-108.
CrossRef - Zhang, M.; Lan, G.; Qiu, H.; Zhang, T.; Li, W.; Hu, X. Environmental Science and Pollution Research., 2019, 26, 3803-3813.
CrossRef - Bode-Aluko, C. A.; Pereao, O.; Ndayambaje, G.; Petrik, L. Water, Air, & Soil Pollution., 2017, 228, 1-11.
CrossRef - Babakhani, A.; & Sartaj, M. Journal of Environmental Chemical Engineering., 2022, 10(2), 107147.
CrossRef - Zhang, L.; Zeng, Y.; Cheng, Z. Journal of Molecular Liquids., 2016, 214, 175-191.
CrossRef - Sivkumar, V.; Kaliappan,S.; Patil,P. Springer link., 2023, 15.
CrossRef - Kumari, Anju.; Kumar, Chnadra prakash.; Kumar,Ganesh.; Sarita, Mukesh and Chowdhary,V. Int. J. of pure and applied chem.,2023, 39,2.

This work is licensed under a Creative Commons Attribution 4.0 International License.










