Pyrazole and its Derivatives: An Excellent N- Hetrocycle with Wide Range of Biological Applications
Munish Kumar and Sharad Kumar Panday*
Department of Chemistry, Faculty of Engineering and Technology M.J.P. Rohilkhand University, Bareilly, U.P., India.
Corresponding Author E-mail: skpanday@mjpru.ac.in
DOI : http://dx.doi.org/10.13005/ojc/380306
Article Received on : 18 Feb 2022
Article Accepted on : 05 Jun 2022
Article Published : 29 Jun 2022
Reviewed by: Dr. Maher Khalid
Second Review by: Dr. Vidya V.G.
Final Approval by: Dr. Paul Metilda
The pyrazole derivatives have been recognized as a unique heterocyclic molecule exerting broad range of biological activities such as analgesic, anti-viral, anti-histaminic, anti-microbial, anti-tumor, insecticides fungicides, anti-depressant, antipyretic, anti-inflammatory, angiotensin converting enzyme (ACE) inhibitory and estrogen receptor (ER) ligand activity etc. Pyrazoles also find applications in agrochemical and pharmaceutical industry. Pyrazoles have different chemical properties which may be attributed due to the effect of particular N-atoms present in pyrazole molecule. N-Atom present at position-2 having non Huckel lone pair is more reactive towards electrophiles while N-atom present at position-1 is unreactive. However, in the presence of strong base, the proton from N-atom at position-1 is abstracted thereby providing pyrazole anion after deprotonation, which in turn increases reactivity towards the electrophiles. There are wide range of drugs available in the market possessing pyrazole nuclei. The present manuscript is aimed to describe major developments achieved till date towards the synthesis and biological applications of pyrazole/pyrazole derivatives and is likely to be beneficial to the researchers working in the area.
KEYWORDS: Download this article as:| Copy the following to cite this article: Kumar M, Panday S. K. Pyrazole and its Derivatives: An Excellent N- Hetrocycle with Wide Range of Biological Applications. Orient J Chem 2022;38(3). |
| Copy the following to cite this URL: Kumar M, Panday S. K. Pyrazole and its Derivatives: An Excellent N- Hetrocycle with Wide Range of Biological Applications. Orient J Chem 2022;38(3). Available from: https://bit.ly/3y5LNYk |
Introduction
Pyrazole1 a five- membered planar N-heterocyclic compound which is aromatic in nature having 4π-electrons and one unshaired pair of electrons delocalized with π-electrons. Pyrazole ring structure contains three carbon atoms along with two nitrogen atoms present in adjacent positions. The lone pair of first N-atom participates in delocalization with π-electrons while the other lone pair present on the second N-atom is non-Huckel lone pair and due to that lone-pair pyrazole shows lewis basicity with PKb11.5 . The pyrazole is represented by the following structure.
 |
Figure 1: Structure of pyrazole1. |
Back Ground and Medicinal Importance
In 1883 Ludwig Knorr was first to abbreviate the term of pyrazole. The first natural pyrazole is 1-pyrazole-alanine which was isolated in 1959 from watermelon seeds1,2. Pyrazoles are also known as azoles3 and pyrazoles act as ligands for different Lewis acids3. The pyrazole derivatives have shown a long range of biological activities including antioxident4, anti-viral5, anti-histaminic6, anti-microbial7, anti-tumor8,9, fungicides10, anti-depressant11, antipyretic12, analgesic12, anti-inflammatory12, angiotensin converting enzyme (ACE) inhibitory13, and estrogen receptor (ER) ligand activity14 etc. Pyrazoles also find applications in agrochemical and pharmaceutical industry15. Pyrazoles have different chemical properties which can be described by the effect of particular atoms present in pyrazole molecule. N-Atom at position-2 having non Huckel lone pair is more reactive towards electrophiles while N-atom at position-1 is unreactive16. However in the presence of a strong base, the proton from N-atom at position-1 is abstracted thereby providing pyrazole anion after deprotonation, which in turn increases reactivity towards the electrophiles17. There are wide range of drugs available in the market possessing pyrazole nuclei and few of these are summarized below18-29.
 |
Table 1: Few important drugs possessing pyrazole nuclei18-29. |
Synthesis of Pyrazole and its Derivatives
Taking into amount the wide range of biological activities associated with pyrazole and its derivatives, numerous synthetic strategies are reported for the preparation of pyrazoles/ pyrazole derivatives and few of these selected ones are being described in the present communication. In one of the strategy N-Hetero aryl compound was converted to pyrazole derivative via transhydra zonation or cyclization in the presence of strongly acidic medium. Initial step for the amination of deactivated 5- bromo-2-Methyl pyridine to benzophenone hydrazone was carefully carried out using 1,1’-Bis-(diphenylphosphino)-ferrocene(DPPF) and Palladium(II) acetate (Pd(OAc)2) (Scheme-1)30.
 |
Scheme 1: Synthesis of pyrazole derivative via trans-hydra-zonation or cyclization30. |
The synthetic strategy for 3,5-disubstituted pyrazoles have been achieved by the condensation of 1,3-dienophilic synthons such as propargylic ketones (Scheme-2)31.
 |
Scheme 2: Synthesis of 3,5-disubstituted pyrazoles through the condensation of 1,3-dienophilic synthons31. |
p-Nitrobenzaldehyde phenylhydrazone was condensed with methylglyoxal to furnish the 4-hydroxy-3-para-nitrophenyl-5-methyl-N-phenylpyrazol (Scheme-3)32.
 |
Scheme 3: Synthesis of 4-hydroxy-3-para-nitrophenyl-5-methyl-N-phenyl-pyrazole32. |
Dzvinchuk et al. explored a strategy for the synthesis of Pyrazole derivatives from (Z)-3-Acetyl-2-methyl-2,3-dihydro-1,4-benzodioxin-2-ol(Scheme-4)33.
 |
Scheme 4: Synthesis of Pyrazole derivative from |
Y.A. Azev et al. synthesized Pyrazole derivatives from the condensation of 1-amino-6,7-difluoro-4-oxoquinolyl-3-ethylcarboxylate with acetoacetone (Scheme-5)34.
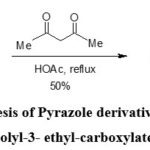 |
Scheme 5: Synthesis of Pyrazole derivative from 1-amino-6, 7-difluoro-4-oxoquinolyl-3- ethyl-carboxylate with aceto-acetone34. |
Katritzky et al. reported a regioselective condensation of α-benzotriazolylenones with phenyl or methyl-hyrdrazines and pyrazolines as the intermediate which gave 1-methyl(aryl)-3-phenyl-5-alkyl(aryl)pyrazoles in basic medium (Scheme-6)35.
 |
Scheme 6: Synthesis of 1-methyl(aryl)-3-phenyl-5-alkyl(aryl)pyrazoles by condensation of α-benzotriazolylenones35. |
Mourea and Delange et al. reported the cyclo condensation of acetylenic ketones and hydrazine derivatives to form pyrazoles derivatives. The saidmethodology was investigated for almost more than a century back in 1901. However the two isomers were reported to be obtained (Scheme-7)36.
 |
Scheme 7: Cyclo- condensation of acetylenic ketones and hydrazine derivatives leading to pyrazole derivatives36. |
1,3- di substituted pyrazoles can also be obtained from the reaction of diaryl-hyrazones and 1,2-diols in presence of Ferric chloride(FeCl3) (Scheme-8)37.
 |
Scheme 8: Synthesis of 1,3- di substituted pyrazoles37. |
Baldwin et al. reported the synthesis of two isomeric pyrazoles by the reaction of Phenyl hydrazine with diacetylene Ketones in ethyl alcohol (Scheme-9)38.
 |
Scheme 9: Synthesis of two isomeric pyrazoles by the reaction of Phenyl hydrazine with diacetylene Ketones38. |
1,4-disubstitued pyrazoles have been synthesized from 1,3-diols and aryl hydrazine through Ruthenium catalyzed condensation (Scheme-10)39.
 |
Scheme 10: Synthesis of 1,4-disubstituedpyrazoles by the reaction of 1,3-diols with aryl hydrazine through Ruthenium catalyzed condensation39. |
Guojing and wang et al. synthesized 3-trifluoromethyl pyrazole via cyclization / trifluoromethylation of phenyl hydrazine and acetylenic Ketones using hypervalent iodine under transition metal free conditions, which gave Togni reagent and subsequently furnished 3-trifluromethyl pyrazole in high yields (70%) (Scheme-11)40.
 |
Scheme 11: Synthesis of 3-trifluoromethyl pyrazole via cyclization / trifluoromethylation of phenyl hydrazine and acetylenic ketones40. |
3,5- disubstituted 1H-pyrazoles were synthesized by the cyclo-addition reaction of tosylhydrazones of aromatic aldehydes with terminal alkynes (Scheme-12)41.
 |
Scheme 12: Synthesis of 3,5- disubstituted 1H-pyrazoles41 |
Bishop et al. explored the synthesis of 3,5-di aryl pyrazoles by the cyclo-condensation of acetylenic ketones and aryl hydrazines or methyl hydrazines in ethyl alcohol which afforded two isomeric pyrazole derivatives (Scheme-13)42.
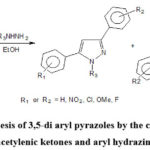 |
Scheme 13: Synthesis of 3,5-di aryl pyrazoles by the cyclo-condensation of acetylenic ketones and aryl hydrazines42. |
Reddy et al. reported an easy approach for the synthesis of 3,5-disubstituted 1H-pyrazole from propargylic alcohols via an acid-catalyzed propargylation followed by cyclization of N,N-disubstituted hydrazines under basic conditions (Scheme-14)43.
 |
Scheme 14: Synthesis of 3,5-disubstituted 1H-pyrazole from propargylic alcohols via acid-catalyzed propargylation followed by cyclization of N, N-disubstitutedhydrazines43. |
Bhat et al. reported the synthesis of pyrazole derivatives by the reaction of β-aryl chalcones and H2O2 furnishing epoxides. The addition of hydrated hydrazine to it, followed by dehydration provided 3,5-diaryl-1H-pyrazole (Scheme-15)44.
 |
Scheme 15: Synthesis of pyrazole derivatives by the reaction of β-aryl chalcones and H2O2 followed by dehydration44. |
Ding et al. reported the synthesis of 3,5-disubstituted pyrazoles from Michael acceptors and methyl hydrazine under mild conditions. The reaction proceeded through Visible Light Photoredox Catalysis (VLPC) (Scheme-16)45.
 |
Scheme 16: Synthesis of 3,5-disubstituted pyrazoles from Michael acceptors and methyl hydrazine45. |
Huang and Katzenellenbogen et al. described the synthesis of 4-alkyl-1,3,5-triaryl pyrazoles by the condensation of α,β-ethylenic ketons with hydrazines in N,N-dimethyl formamide providing pyrazoline as intermediate. The alkylation of pyrazoline in presence of lithium diisopropyl amide(LDA) furnished 4-alkyl-1,3,5-triarly pyrazole (Scheme-17)46.
 |
Scheme 17: Synthesis of 4-alkyl-1,3,5-triaryl pyrazoles by the condensation of α, β-ethylenic ketones with hydrazines46. |
Zhang et al. reported the synthesis of polysubstituted pyrazoles from α,β-unsaturated carbonyls (aldehyde and ketone) and salts of hydrazine (Scheme-18)47.
 |
Scheme 18: Synthesis of poly-substituted pyrazoles from α, β-unsaturated carbonyls and salts of hydrazine47. |
Jiany et al. described an efficient method for the synthesis of 3,5-disubstituted-N-phenyl pyrazole by the cyclocondensation of phenylhydrazine and α,β ethylenic ketone in presence of molecular Iodine(I2) (Scheme-19)48.
 |
Scheme 19: Synthesis of 3,5-disubstituted-N-phenyl pyrazole by the cyclo-condensation of phenyl hydrazine and α, β-ethylenic ketone48. |
Tang et al. reported the reaction of terminal alkynes and N-alkylated tosylhydrazones in the presence of AlCl3,thereby affording 1,3,5-trisubstituted pyrazoles in good yields (Scheme-20)49.
 |
Scheme 20: Synthesis of 1,3,5-trisubstituted pyrazoles49. |
He and Chenet al. reported the synthesis of pyrazole derivatives by the cycloaddition reaction of phenyl propargyl and ethyl α-diazoacetate in presence of triethylamine as base and triflate as a catalyst (Scheme-21)50.
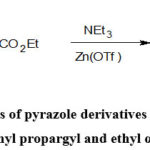 |
Scheme-21: Synthesis of pyrazole derivatives by the cycloaddition reaction of phenyl propargyl and ethyl α-diazoacetate50. |
Girish & Kumar et al. synthesis 1,3,5,-tri substituted pyrazole by the condensation of ethyl acetoacetate with phenylhydrazine (Scheme-22)51
 |
Scheme 22: synthesis 1,3, 5, – tri substituted pyrazole by condensation. |
Jiang et al. developed the synthesis of regioisomer of pyrazole derivatives from the cyclisation of α-diazoarylacetate and propionate followed by prototropic Rearrangement (Scheme-23)52.
 |
Scheme 23: Synthesis of regio-isomer of pyrazole derivatives by the cyclisation of α-diazo-arylacetate and propionate followed by proto tropic rearrangement52. |
Y. Kong et al. synthesized 1,3,5-trisubstituted pyrazoles from terminal alkynes and N-alkylated tosylhydrazones. This methodology provided trisubstituted pyrazoles with high regioselectivity (Scheme-24)53.
 |
Scheme 24: Synthesis of 1,3,5-trisubstituted pyrazoles53. |
Harigae and Moriyam et al. synthesized 3,5-substituted pyrazole in high yields by the reaction of terminal alkynes with hydrated hydrazine furnishing 3,5-substituted pyrazole (Scheme-25)54.
 |
Scheme 25: Synthesis of 3,5-substituted pyrazole by the reaction of terminal alkynes with hydrated hydrazine54. |
Zhang et al. developed an easy approach for the synthesis of trisubstituted 1H-pyrazoles from vinyl azide, tosylhydrazine and aldehydes using of base (Scheme-26)55.
 |
Scheme 26: Synthesis of trisubstituted 1H-pyrazoles from vinyl azide, tosyl-hydrazine and aldehydes55. |
Lizuka et al. described the palladium catalyzed carbonylation reaction of acetylenic acids with aryl iodides using of Molybdenum hexacarbonyl(Mo(CO)6) toget 1,3,5-trisubstituted pyrazole in good yields (Scheme-27)56.
 |
Scheme 27: Synthesis of 1,3,5-trisubstituted pyrazole56. |
Heller et al. explored a synthetic methodology for trisubstituted pyrazoles from by 1,3-diketones which were obtained from acid chloride and ketone (Scheme-28)57.
 |
Scheme 28: Synthesis of tri substituted pyrazoles from 1,3-diketones57. |
Kovacs and Co- workers reported a new route for the synthesis of 3,5-disubstituted pyrazoles by the coupling reaction of an oxime with alkyne in the presence of Cu/Fe providing β-aminoenone which on addition with hydrazine in DMF provided 3,5-disubstitued pyrazoles in satisfactory yields(70%) (Scheme-29)58.
 |
Scheme 29: Synthesis of 3,5-disubstituted pyrazoles by the coupling reaction58. |
Gosselin et al. synthesized N-aryl-3,5-substituted pyrazoles by the condensation of 1,3-diketones and arylhydrazines at room temperature using N,N-dimethylacetamide as solvent (Scheme-30)59.
 |
Scheme 30: Synthesis of N-aryl-3,5-substituted pyrazoles by the condensation of 1,3-diketones with arylhydrazines59. |
Dang and Fischer et al. developed a method for the synthesis of pyrazole-3-carboxylate by cyclization of diethyl dioxalate and hydrazones furnishing pyrazole-3-carboxylate in 53% yield (Scheme-31)60.
 |
Scheme 31: Synthesis of pyrazole-3-carboxylate by cyclization of diethyl di oxalate and hydrazones60. |
Ohtsuka and Uraguchi et al. Synthesis of 1,3,4,5-tetra substituted pyrazole derivative from condensation of phenyl hydrazine with 2-(trifluoromethyl)-1,3-diketone in solvent of ethanol. (Scheme-32)61.
 |
Scheme 32: Synthesis of 1,3,4,5-tetra substituted pyrazole derivative from condensation. |
Lokhande and Hasanzadeh et al. synthesized 4-formyl pyrazole by the condensation of hydrazine in presence of Phosphorus oxychloride(POCl3) in DMF (Scheme-33)62.
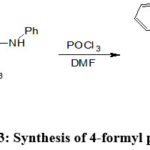 |
Scheme 33: Synthesis of 4-formyl pyrazole62. |
Fan and Lei et al. explored an efficient method for the synthesis of tri-substituted pyrazoles from α-bromo ketones and hydrazones. The reaction involved radical addition reaction followed by intramolecular cyclisation (Scheme-34)63.
 |
Scheme 34: Synthesis of tri-substituted pyrazoles from α-bromo ketones and hydrazones63. |
Aggarwal and Vicente et al. developed a process in which diazo derivatives formed in situ from aldehyde and tosylhydrazines by 1,3-dipolar cycloaddition reaction in between diazo compound & terminal alkynes and N-Vinylimidazole furnishing corresponding pyrazole derivatives (Scheme-35)64.
 |
Scheme 35: 1,3-Dipolar cyclo-addition reaction in between diazo compound & terminal alkynes and N-vinyl imidazole leading to pyrazoles derivatives64. |
Kumar and Yadav et al. reported the synthesis of substituted pyrazoles by the reaction of 1,3-bisaryl monothio-1,3-diketone and arylhydrazines in ethyl alcohol (Scheme-36)65.
 |
Scheme 36: Synthesis of substituted pyrazoles by the reaction of 1,3-bisaryl monothio-1,3-diketone and arylhydrazines65. |
Many methods for the synthesis of pyrazoles by the reaction of hydrazines with heterocycle compounds have been reported (Scheme-37)66-70.
 |
Scheme 37: Different strategies for the synthesis of pyrazoles by the reaction of hydrazines with heterocyclic compounds66-70. |
Sha et al. synthesized 3,5-diaryl-4-bromo-1H-pyrazoles from alkenyl bromides and diazo compounds by 1,3-dipolar cyclo-addition, where other isomeric products were also obtained (Scheme-38)71.
 |
Scheme 38: Synthesis of 3,5-diaryl-4-bromo-1H-pyrazoles from alkenyl bromides and diazocompounds71. |
Xie and Chen et al. reported the synthesis of pyrazoles by Suzuki coupling reactions(Scheme-39)72.
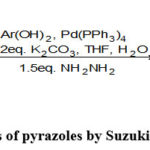 |
Scheme 39: Synthesis of pyrazoles by Suzuki coupling reactions72. |
Gerstenberger et al. synthesized N-aryl 3,4,5-trisubstituted pyrazoles from aryl halide, di-tert-butylazodicarboxlate (Boc) and 1,3-dicarbonyl compounds (Scheme-40)73.
 |
Scheme 40: Synthesis of N-aryl 3,4,5-trisubstituted pyrazoles73. |
Liham and Saripinar et al. reported the condensation of furan 2,3-dione with aryl hydrazine providing pyrazole derivatives.Similarly Sener et al. reported the condensation of furan-2,3-dione with N-benzylidene-N’-(4-nitrophenyl) hydrazine furnishing 4-benzoyl-1-(4-nitropphenyl)-5-phenyl-1H-pyrazole-3-carboxylic acid (Scheme-41)74-75.
 |
Scheme 41: Synthesis of pyrazoles by the condensation of furan 2,3-dione with aryl hydrazine74-75. |
Ahmed and Kobayashi et al. reported an efficient method for the synthesis of N-methyl 3,5-disubstituted pyrazoles from terminal alkynes, methyl hydrazine and aryl halide (Scheme-42)76.
 |
Scheme 42: Synthesis of N-methyl 3,5-disubstituted pyrazoles from terminal alkynes, methyl hydrazine and aryl halide76. |
Groseli et al. developed a new method for the preparation of pyrazole derivatives by the following cyclo-addition reaction (Scheme-43)77.
 |
Scheme 43: Synthesis of pyrazole derivatives by cycloaddition reaction77. |
Deng et al. reported a of highly regioselective synthesis of tetra-substituted pyrazoles from nitro-olefins and hydrazones in the presence of strong base (Scheme-44)78.
 |
Scheme 44: Synthesis of tetra-substituted pyrazoles from nitro-olefins and hydrazones78. |
When 4-trifluoroacetyl-1,3-oxazolium-5-olates were heated with phenylhydrazine, it provided 5-trifluoromethyl pyrazole derivative a procedure developed by Kawase and Koiwai et al (Scheme-45)79.
 |
Scheme 45: Synthesis of 5-trifluoromethyl pyrazole derivatives by reaction of 4-trifluoroacetyl-1,3-oxazolium-5-olate with phenylhydrazine79. |
Deng et al. reported the synthesis of 1,3,4,5-tetra-substituted pyrazoles and 1,3,5-tri substituted pyrazoles with high regioselectivity from N-aryl hydrazones and nitro-olefins in the presence of ethyl glycol at 120 0C (Scheme-46)80.
 |
Scheme 46: Regioselective synthesis of 1,3,4,5-tetra-substituted pyrazoles and 1,3,5-trisubstituted pyrazoles80. |
When tetrazolylacroleins were allowed to undergo reaction with fumaronitrile at 140oC in xylene it provided pyrazole as reported by Simoni et al (Scheme-47)81.
 |
Scheme 47: Synthesis of pyrazole by reaction of |
Deng et al. reported the synthesis of tetra substituted pyrazoles by the reaction of N-substituted hydrazones with nitro-olefins in high yields (Scheme-48)82.
 |
Scheme 48: Synthesis of tetra substituted pyrazoles by the reaction of N-substituted hydrazones with nitro-olefins82. |
Rykowski and Branowska et al. explored an efficient method for the synthesis of pyrazoles by the condensation of 3-chloro-6-phenyl-1,2,4-triazines with α-chlorosulfonyls in DMSO using Potassium hydroxide as base (Scheme-49)83.
 |
Scheme 49: Synthesis of pyrazoles by the condensation of 3-chloro-6-phenyl-1,2,4-triazines with α-chlorosulfonyls83. |
Wen and Tang et al. synthesized various highly functionalized pyrazoles by Pt-catalyzed (3,3)-sigmatropic rearrangement of N-propargyl hydrazones (Scheme-50)84.
 |
Scheme 50: Synthesis of pyrazoles by Pt-catalyzed (3,3)- sigma tropic rearrangement of N-propargyl hydrazones84. |
Ferfra and Ahabchane et al. described a method for the synthesis of pyrazoles by the reaction of benzodiazepine-2-thiones with hydrazine (Scheme-51)85.
 |
Scheme 51: Synthesis of pyrazoles by the reaction of benzodiazepine-2-thiones with hydrazine85. |
Hu and Chen et al. synthesized various tetrasubstituted pyrazoles by the ruthenium-catalyzed oxidative coupling reaction in presence of O2 as an oxidant (Scheme-52)86.
 |
Scheme 52: Synthesis of tetra- substituted pyrazoles by the ruthenium-catalyzed oxidative coupling reaction86. |
Pfeffer et al. reported 5-amino-pyrazoles which were obtained by heating 3-methyl-6H-1,3,4-thiadiazine acetic acid (Scheme-53)87.
 |
Scheme 53: Synthesis of 5-amino-pyrazoles from 3-methyl-6H-1,3,4-thiadiazine acetic acid87. |
Martin et al. prepared pyrazole derivatives by Cu-catalyzed C-N coupling reaction (Scheme-54)88.
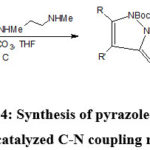 |
Scheme 54: Synthesis of pyrazole derivatives by Cu-catalyzed C-N coupling reaction88. |
When nitropyrimidine was allowed to undergo reaction with aryl hydrazines in methyl alcohol at 25oC temperature, it furnished 4-nitro-3,5-diamino-pyrazole in good yields (Scheme-55)89.
 |
Scheme 55: Synthesis of 4-nitro-3,5-diamino-pyrazole in good yields89. |
Q. Zhang et al. synthesized pyrazole derivatives in good yields by cyclo-addition of allylic carbonate and arylazosulfones in presence of tri-butylphosphine(PBu3) under mild reaction conditions (Scheme-56)90.
 |
Scheme 56: Synthesis of pyrazole derivatives in good yields by cyclo-addition of allylic carbonate and arylazosulfones90. |
Suen and Hope et al. described a method to prepare a series of pyrazole derivatives by the condensation of thietanone & 1,2,4,5-tetrazine in alcohol in the presence of KOH(Scheme-57)91.
 |
Scheme 57: Synthesis of pyrazole derivatives by the condensation of thietanone & 1,2,4,5-tetrazine in alcohol91. |
Jiang et al. synthesized 4-substituted 1,5-diaryl pyrazole-3-carboxylic acids via claisen condensation-Knorr reaction which was carried out in the presence of Lithium chloride (LiCl) and Sodium methoxide (NaOMe) (Scheme-58)92.
 |
Scheme 58: Synthesis of 4-substituted 1,5-diaryl pyrazole-3-carboxylic acids via claisen condensation-Knorr reaction92. |
Synthesis of Pyrazole Derivatives Through Green Synthesis
The pyrazole/substituted pyrazoles have also been frequently employed in green synthesis leading to formation of various pyrazole derivatives possessing diversified biological activities93,94 The Claisen–Schmidt condensation of substituted 1,3-diphenyl-1H-pyrazole-4- carbaldehydes and 1-(2,4-dimethoxy-phenyl)- ethanone led to the development of novel chalcones, 1-(2,4-dimethoxy-phenyl)-3-(1,3-diphenyl1H-pyrazol-4-yl)-propenone. The reaction was carried out at room temperature in ethanol. Out of the several derivatives synthesized it was concluded that most of the compounds were nontoxic except compound g (Scheme-59)95.
 |
Scheme 59: Synthesis of pyrazole derivatives through green synthesis. |
The reaction of dialkyl acetylenedicarboxylates, isocyanides and the 1,2-dibenzoylhydrazines with tetrabutylammonium bromide (TBTB) was carried out, where tetrabutylammonium bromide (TBTB) was used as an environment friendly organic ionic salt as well as high polar reaction medium under solvent free conditions at room temperature. This green synthetic approach was explored to get highly functionalized pyrazole derivative (Scheme-60)96
 |
Scheme 60: The green synthetic approach for the synthesis of highly functionalized pyrazole derivatives using tetra-butyl ammonium bromide (TBTB) as an environment friendly organic ionic salt. |
Pharmaceutical Applications
Derivatives of pyrazole are reported to be physiologically and pharmacologically active and these find use in various drugs for the treatment of several diseases. Hence pyrazole derivatives are biologically and pharmaceutically quite indispensable. The compounds having pyrazole nuclei have wide uses in agro-chemistry and pharmaceuticals. Various potential biological activities have been reported. The biological evaluation such as anti-bacterial activities of pyrazole derivatives has been done in an exhaustive manner, where a series of pyrazole derivatives were screened for the activities against the gram negative bacteria such as Pseudomonas piosineus, E.coli etc. applying agar plate diffusion technique97 and gram positive bacteria such as S. aureus, S. albus etc98. Pyrazole derivatives have also been found to have anti-HIV activity which involved the susceptible human host cells and have been tested for their anti-viral activity99 particularly AIDS. Pyrazoles also act as herbicidal100, insecticidal101, anti-schistosomal102 and anticancer100-104 properties. 1-N-arylpyrazole derivatives show sedative, analgesic and hypnotic activities105-107. Different pyrazoles exhibit different biological activities as shown in the table given below.
Conclusion
Based on the literature reports Pyrazole and its derivatives are undoubtedly one of the most important class of organic heterocyclic possessing wide range of biological activities some of the representatives such as anti-histamine, anti-viral, anti-tumor, anti-microbial, anti-bacterial, anti-pyretic, anti-depressant, anti-inflammatory, anti-cancer, fungicides, insecticides, analgesic etc. have been summarized in the present communication. However there is still a used to explore a cheap and easy synthetic strategy for the synthesis of such an important molecule list wise the biological application and medicinal importance in wide spectrum is yet to be investigated to prove the pyrazole/ pyrazole derivatives as one of the important tool for organic/ Medicinal chemist and to exploit further the chemistry of pyrazole for the welfare of mankind over the globe.
Acknowledgement
The Principal author is thankful to TEQIP-III (MHRD) for financial support in form of minor research project and first author Munish Kumar is thankful to Council of Scientific and Industrial Research, India for providing financial assistance in form of Junior Research Fellowship and Senior Research Fellowship.
Conflict of Interest
The author declares that no conflict of interest.
Funding Sources
There is no funding source.
References
- Perrin, D. D. Dissociation Constants of Organic Bases Aqueous Solution. 1. Google Scholar. 1972.
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles. 2003, 2, 61-79.
CrossRef - Mukherjee, R. Coordination Chemistry Reviews. 2000, 203, 151-218.
CrossRef - Fustero, S.; Sanchez-Rosello, M.; Barrio, P.; Simon-Fuentes, A. Chemical reviews. 2011, 111, 6984-7034.
CrossRef - Larsen, J. S.; Zahran, M. A.; Pedersen, E. B.; Nielsen, C. Monatshefte für Chemie/Chemical Monthly. 1999, 130, 1167-1173.
CrossRef - Yıldırım, I.; Ozdemir, N.; Akçamur, Y.; Dinçer, M.; Andaç, O. Acta Crystallographica Section E: Structure Reports Online. 2005, 61, 0256-0258.
CrossRef - Pimerova, E. V.; Voronina, E. V. Pharm. Chem. J. 2001, 35, 602-604.
CrossRef - Park, H. J.; Lee, K.; Park, S. J.; Ahn, B.; Lee, J. C.; Cho, H.; Lee, K. I. Bioorganic & medicinal chemistry letters. 2005, 15, 3307-3312.
CrossRef - Bouabdallah, I.; M’Barek, L. A.; Zyad, A.; Ramdani, A.; Zidane, I.; Melhaoui, A. Natural product research. 2006, 20, 1024-1030.
CrossRef - Chu, C. K.; Cutler, S. J. Journal of heterocyclic chemistry. 1986, 23, 289-319.
CrossRef - Bailey, D. M.; Hansen, P. E.; Hlavac, A. G.; Baizman, E. R.; Pearl, J.; DeFelice, A. F.; Feigenson, M. E. Journal of medicinal chemistry. 1985, 28, 256-260.
CrossRef - Valentina, P.; Ilango, K.; Kathiravan, M. K. Archives of pharmacal research. 2016, 39, 1382-1390.
CrossRef - (a). Panday, S. K.; Dikshit, M.; Dikshit, D. K. Medicinal chemistry research. 2009, 18, 566-578. (b). Prasad, J.; Pathak, M. B.; Panday, S. K. Medicinal Chemistry Research. 2012, 21, 321-324.
CrossRef - Katz, A. M., Pearson, C. M., & Kennedy, J. M. (1965). A clinical trial of indomethacin in rheumatoid arthritis. Clinical Pharmacology & Therapeutics, 6, 25-30.
CrossRef - Katritzky, A. R.; Rees, C. W.; Comprehensive heterocyclic chemistry. 1984, Pergamon Press.
CrossRef - Yerragunta, V.; Suman, D.; Anusha, V.; Patil, P.; Naresh, M. PharmaTutor. 2014, 2, 40-48.
- Bansal, R. K. Heterocyclic chemistry. 2008, New Age International.
- Kim, S. H.; Na, S. Y.; Ha, H. J.; Lee, W. K. Asian Journal of Organic Chemistry. 2019, 8, 1680-1686.
CrossRef - MG, P. Saunders handbook of veterinary drugs: small and large animal. Graham B, editor. 2016, 3.
- Tallett, A. J.; Blundell, J. E.; Rodgers, R. J. Psychopharmacology. 2007, 195, 27-39.
CrossRef - Goddard, C. J. Journal of heterocyclic chemistry. 1991, 28, 1607-1612.
CrossRef - Steinbach, G.; Lynch, P. M.; Phillips, R. K.; Wallace, M. H.; Hawk, E.; Gordon, G. B.; Kelloff, G. New England Journal of Medicine. 2000, 342, 1946-1952.
CrossRef - Uslaner, J. M.; Parmentier-Batteur, S.; Flick, R. B.; Surles, N. O.; Lam, J. S.; Mc Naughton, C. H.; Hutson, P. H. Neuropharmacology. 2009, 57, 531-538.
CrossRef - Friedrich, G.; Rose, T.; Rissler, K. Determination of lonazolac and its hydroxy and O-sulfated metabolites by on-line sample preparation liquid chromatography with fluorescence detection. Journal of Chromatography B. 2002, 766, 295-305.
CrossRef - Hampp, C.; Hartzema, A. G.; Kauf, T. L. Value in Health. 2008, 11, 389-399.
CrossRef - Spitz, I. M.; Novis, B. H.; Ebert, R.; Trestian, S.; LeRoith, D.; Creutzfeldt, W. Metabolism. 1982, 31, 380-382.
CrossRef - Luttinger, D.; Hlasta, D. J. Annual Reports in Medicinal Chemistry. 1987, 22, 21-30.
CrossRef - Tsutomu, K.; Toshitaka, N. Neuropharmacology. 1978, 17, 249-256.
CrossRef - García-Lozano, J.; Server-Carrió, J.; Escrivà, E.; Folgado, J. V.; Molla, C.; Lezama, L. Polyhedron. 1997, 16, 939-944.
CrossRef - Haddad, N.; Salvagno, A.; Busacca, C. Tetrahedron letters. 2004, 45, 5935-5937.
CrossRef - Wang, X. J.; Tan, J.; Grozinger, K. Tetrahedron Letters. 2000, 41, 4713-4716.
CrossRef - Iwata, S.; Namekata, J.; Tanaka, K.; Mitsuhashi, K. J. Heterocyclic, Chem. 1991, 28, 1971.
CrossRef - Dzvinchuk, I. V.; Lozinskii, M. O. Chemistry of Heterocyclic Compounds. 2001, 37, 459-462.
CrossRef - Azev, Y. A.; Lork, E.; Gabel, D.; Duelcks, T. Mendeleev Communications. 2003, 13, 184-185.
CrossRef - Katritzky, A. R.; Wang, M.; Zhang, S.; Voronkov, M.V.; Steel, P. J. The Journal of organic chemistry. 2001, 66, 6787-6791.
CrossRef - Moureu, C.; Delange, R. Bull. Soc. Chim. Fr. 1901, 25, 302-313.
- Panda, N.; Jena, A. K. The Journal of organic chemistry. 2012, 77, 9401-9406.
CrossRef - Baldwin, J. E.; Pritchard, G. J.; Rathmell, R. E. Journal of the Chemical Society, Perkin Transactions 1. 2001, 22, 2906-2908.
CrossRef - Schmitt, D.C.; Taylor, A.P.; Flick, A.C.; Kyne Jr, R. E. Organic letters. 2015, 17, 1405-1408.
CrossRef - Ji, G.; Wang, X.; Zhang, S.; Xu, Y.; Ye, Y.; Li, M.; Wang, J. Chemical Communications. 2014, 50, 4361-4363.
CrossRef - Wu, L. L.; Ge, Y. C.; He, T.; Zhang, L.; Fu, X. L.; Fu, H. Y.; Li, R. X. Synthesis. 2012, 44, 1577-1583.
CrossRef - Bishop, B. C.; Brands, K. M.; Gibb, A. D.; Kennedy, D. J. Synthesis. 2004, 01, 43-52.
CrossRef - Reddy, C. R.; Vijaykumar, J.; Gree, R. Synthesis. 2013, 45, 830-836.
CrossRef - Bhat, B. A.; Puri, S. C.; Qurishi, M. A.; Dhar, K. L.; Qazi, G. N. Synthetic Communications. 2005, 35, 1135-1142.
CrossRef - Ding, Y.; Zhang, T.; Chen, Q. Y.; Zhu, C. Organic letters. 2016, 18, 4206-4209.
CrossRef - Huang, Y. R.; Katzenellenbogen, J. A. Organic letters. 2000, 2, 2833-2836.
CrossRef - Zhang, X.; Kang, J.; Niu, P.; Wu, J.; Yu, W.; Chang, J. The Journal of organic chemistry. 2014, 79, 10170-10178.
CrossRef - Ponnala, S.; Prasad Sahu, D. Synthetic communications. 2006, 36, 2189-2194.
CrossRef - Tang, M.; Wang, Y.; Wang, H.; Kong, Y. Synthesis. 2016, 48, 3065-3076.
CrossRef - He, S.; Chen, L.; Niu, Y. N.; Wu, L. Y.; Liang, Y. M. Tetrahedron Letters. 2009, 50, 2443-2445.
CrossRef - Girish, Y. R.; Kumar, K. S. S.; Manasa, H. S.; Shashikanth, S. Journal of the Chinese Chemical Society. 2014, 61, 1175-1179.
CrossRef - Jiang, N.; Li, C. J. Chemical communications. 2004, 4, 394-395.
CrossRef - Kong, Y.; Tang, M.; Wang, Y. Organic letters. 2014, 16, 576-579.
CrossRef - Harigae, R.; Moriyama, K.; Togo, H. The Journal of organic chemistry. 2014, 79, 2049-2058.
CrossRef - Zhang, G.; Ni, H.; Chen, W.; Shao, J.; Liu, H.; Chen, B.; Yu, Y. Organic letters. 2013, 15, 5967-5969.
CrossRef - Iizuka, M.; Kondo, Y. 2007, Palladium‐Catalyzed Alkynylcarbonylation of Aryl Iodides with the Use of Mo (CO) 6 in the Presence of tBu3P Ligand.
CrossRef - Heller, S. T.; Natarajan, S. R. Organic letters. 2006, 8, 2675-2678.
CrossRef - Kovacs, S.; Novak, Z. Tetrahedron. 2013, 69, 8987-8993.
CrossRef - Gosselin, F.; O’Shea, P. D.; Webster, R. A.; Reamer, R. A.; Tillyer, R. D.; Grabowski, E. J. Synlett. 2006, 19, 3267-3270.
CrossRef - Dang, T. T.; Dang, T. T.; Fischer, C.; Görls, H.; Langer, P. Tetrahedron. 2008, 64, 2207-2215.
CrossRef - Ohtsuka, Y.; Uraguchi, D.; Yamamoto, K.; Tokuhisa, K.; Yamakawa, T. Tetrahedron. 2012, 68, 2636-2649.
CrossRef - Lokhande, P.; Hasanzadeh, K.; Konda, S. G. European journal of Chemistry. 2011, 2, 223-228.
CrossRef - Fan, X. W.; Lei, T.; Zhou, C.; Meng, Q. Y.; Chen, B.; Tung, C. H.; Wu, L. Z. The Journal of organic chemistry. 2016, 81, 7127-7133.
CrossRef - Illa, O.; Muray, E.; Amsallem, D.; Moglioni, A. G.; Gornitzka, H.; Branchadell, V.; Ortuño, R. M. Tetrahedron: Asymmetry. 2002, 13, 2593-2603.
CrossRef - Kumar, S. V.; Yadav, S. K.; Raghava, B.; Saraiah, B.; Ila, H.; Rangappa, K. S.; Hazra, A. The Journal of organic chemistry. 2013, 78, 4960-4973.
CrossRef - Xie, F.; Cheng, G.; Hu, Y. Journal of combinatorial chemistry. 2006, 8, 286-288.
CrossRef - Arkhipov, V. V.; Garazd, M. M.; Smirnov, M. N.; Khilya, V. P. Chemistry of Heterocyclic Compounds. 2004, 40.
- Sosnovskikh, V. Y.; Barabanov, M. A.; Usachev, B. I. Russian chemical bulletin. 2003, 52, 1758-1767.
CrossRef - Usachev, B. I.; Shafeev, M. A.; Sosnovskikh, V. Y. Russian chemical bulletin. 2004, 53, 2285-2292.
CrossRef - Sosnovskikh, V. Y.; Usachev, B. I.; Sizov, A. Y. Russian chemical bulletin. 2003, 52, 508-510.
CrossRef - Sha, Q.; Wei, Y. Synthesis. 2013, 45, 413-420.
CrossRef - Xie, F.; Cheng, G.; Hu, Y. Journal of combinatorial chemistry. 2006, 8, 286-288.
CrossRef - Gerstenberger, B. S.; Rauckhorst, M. R.; Starr, J. T. Organic letters. 2009, 11, 2097-2100.
CrossRef - Ilhan, I. O.; Saripinar, E.; Akcamur, Y. Journal of heterocyclic chemistry. 2005, 42, 117-120.
CrossRef - Sener, A.; Kasimogullari, R.; Sener, M. K.; Genc, H. Chemistry of Heterocyclic Compounds. 2004, 40, 1039-1046.
CrossRef - Ahmed, M.; Kobayashi, K.; Mori, A. Organic Letters. 2005, 7, 4487-4489.
CrossRef - Grošelj, U.; Drobnic, A.; Recnik, S.; Svete, J.; Stanovnik, B.; Golobic, A.; Golic‐Grdadolnik, S. Helvetica Chimica Acta. 2001, 84, 3403-3417.
CrossRef - Kawase, M.; Koiwai, H.; Yamano, A.; Miyamae, H. Tetrahedron letters. 1998, 39, 663-666.
CrossRef - Deng, X.; Mani, N. S. Organic letters. 2008, 10, 1307-1310.
CrossRef - Simoni, D.; Rondanin, R.; Furnò, G.; Aiello, E.; Invidiata, F. P. Tetrahedron Letters. 2000, 41, 2699-2703.
CrossRef - Deng, X.; Mani, N. S. Organic letters. 2006, 8, 3505-3508.
CrossRef - Rykowski, A.; Branowska, D. Heterocycles. 1996, 10, 2095-2098.
CrossRef - Wen, J. J.; Tang, H. T.; Xiong, K.; Ding, Z. C.; Zhan, Z. P. Organic letters. 2014, 16, 5940-5943.
CrossRef - Ferfra, S.; Ahabchane, N. H.; Garrigues, B.; Essassi, E. M. Comptes Rendus de l’Académie des Sciences-Series IIC-Chemistry. 2001, 4, 905-911.
CrossRef - Hu, J.; Chen, S.; Sun, Y.; Yang, J.; Rao, Y. Organic letters. 2012, 14, 5030-5033.
CrossRef - Pfeiffer, W. D.; Dilk, E.; Rossberg, H.; Langer, P. Synlett, 2003, 15, 2392-2394.
CrossRef - Martín, R.; Rodríguez Rivero, M.; Buchwald, S. L. Angewandte Chemie. 2006, 118, 7237-7240.
CrossRef - Guillard, J.; Goujon, F.; Badol, P.; Poullain, D. Tetrahedron letters. 2003, 44, 5943-5945.
CrossRef - Zhang, Q.; Meng, L. G.; Wang, K.; Wang, L. Organic letters. 2015, 17, 872-875.
CrossRef - Suen, Y. F.; Hope, H.; Nantz, M. H.; Haddadin, M. J.; Kurth, M. J. The Journal of organic chemistry. 2005, 70, 8468-8471.
CrossRef - Jiang, J. A.; Du, C. Y.; Gu, C. H.; Ji, Y. F. Synlett. 2012, 23, 2965-2968.
CrossRef - Capello, C., Fischer, U., & Hungerbühler, K.; What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chemistry. 2007, 9(9), 927-934.
CrossRef - Chanda, A.; Fokin, V.V. Organic Synthesis “On Water”. Chem. Rev.; 2009, 109(2), 725-748.
CrossRef - Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Gawande, N.M.; Khobragade, C.N. Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg. Med. Chem., 2009, 17(24), 8168- 8173.
CrossRef - Soltanzadeh, Z., Imanzadeh, G., Noroozi-Pesyan, N., & Şahin, E.; Green synthesis of pyrazole systems under solvent-free conditions. Green Chemistry Letters and Reviews, 2017, 10(3), 148-153.
CrossRef - Tiwari, A. K.; Mishra, A. K.; Bajpai, A.; Mishra, P.; Sharma, R. K.; Pandey, V. K.; Singh, V. K. Bioorganic & medicinal chemistry letters. 2006, 16, 4581-4585.
CrossRef - Jolly, V. S.; PATHAK, M.; JAIN, R. ChemInform. 1993, 24.
- Weislow, O. S.; Kiser, R.; Fine, D. L.; Bader, J.; Shoemaker, R. H.; Boyd, M. R. JNCI: Journal of the National Cancer Institute. 1989, 81, 577-586.
CrossRef - El Latif, F. M. A.; El Rady, E. A.; Döupp, D. Journal of heterocyclic chemistry. 2003, 40, 57-60.
CrossRef - Hainzl, D.; Cole, L. M.; Casida, J. E. Chemical research in toxicology. 1998, 11, 1529-1535.
CrossRef - Senga, K.; Novinson, T.; Wilson, H. R.; Robins, R. K. Journal of medicinal chemistry. 1981, 24, 610-613.
CrossRef - Wang, F. Q.; Yang, H.; He, B.; Jia, Y. K.; Meng, S. Y.; Zhang, C.; Liu, F. W. Tetrahedron. 2016, 72, 5769-5775.
CrossRef - Wen, J.; Bao, Y.; Niu, Q.; Yang, J.; Fan, Y.; Li, J.; Liu, D. European journal of medicinal chemistry. 2016, 109, 350-359.
CrossRef - Lee, K. Y.; Kim, J. M.; Kim, J. N. Tetrahedron letters. 2003, 44, 6737-6740.
CrossRef - Bratušek, U.; Hvala, A.; Stanovnik, B. Journal of heterocyclic chemistry. 1998, 35, 1281-1284.
- Huang, Y. R.; Katzenellenbogen, J. A. Organic letters. 2000, 2, 2833-2836.
CrossRef - Bekhit, A. A.; Fahmy, H. T.; Rostom, S. A.; Bekhit, A. E. D. A. European journal of medicinal chemistry. 2010, 45, 6027-6038.
CrossRef - Bandgar, B. P., Gawande, S. S., Bodade, R. G., Gawande, N. M., & Khobragade, C. N. Bioorganic & medicinal chemistry. 2009, 17, 8168-8173.
CrossRef - Barsoum, F. F.; Girgis, A. S. European journal of medicinal chemistry. 2009, 44, 2172-2177.
CrossRef - Bekhit, A. A., Abdel-Aziem, T. Bioorganic & medicinal chemistry. 2004, 12, 1935-1945.
CrossRef - Ahsan, M. J.; Saini, V. Beni-Suef University Journal of Basic and Applied Sciences. 2015, 4, 41-46.
CrossRef - Maurya, H. K.; Verma, R.; Alam, S.; Pandey, S.; Pathak, V.; Sharma, S.; Gupta, A. Bioorganic & medicinal chemistry letters. 2013, 23, 5844-5849.
CrossRef - Bondock, S.; Fadaly, W.; Metwally, M. A. European journal of medicinal chemistry. 2010, 45, 3692-3701.
CrossRef - Argade, N. D.; Kalrale, B. K.; Gill, C. H. E-Journal of chemistry. 2008, 5, 120-129.
CrossRef - Naim, M. J.; Alam, O.; Farah Nawaz, M.; Alam, J.; Alam, P. Journal of pharmacy & bioallied sciences. 2016, 8, 2.
CrossRef - Pirol, S.C.; Calıskan, B., Durmaz, I.; Atalay, R.; Banoglu, E. European journal of medicinal chemistry. 2014, 87, 140-149.
CrossRef - Sangani, C. B.; Makawana, J. A.; Zhang, X.; Teraiya, S. B.; Lin, L.; Zhu, H. L. European journal of medicinal chemistry. 2014, 76, 549-557.
CrossRef - Insuasty, B.; Tigreros, A.; Orozco, F.; Quiroga, J.; Abonía, R.; Nogueras, M.; Cobo, J. Bioorganic & medicinal chemistry. 2010, 18, 4965-4974.
CrossRef - Rachakonda, V.; Alla, M.; Kotipalli, S. S.; Ummani, R. European journal of medicinal chemistry. 2013, 70, 536-547.
CrossRef - Mowbray, C. E.; Burt, C.; Corbau, R.; Gayton, S.; Hawes, M.; Perros, M.; Wood, A. Bioorganic & medicinal chemistry letters. 2009, 19(20), 5857-5860.
CrossRef - Bandgar, B. P.; Chavan, H. V.; Adsul, L. K.; Thakare, V. N.; Shringare, S. N.; Shaikh, R.; Gacche, R. N. Bioorganic & medicinal chemistry letters. 2013, 23, 912-916.
CrossRef - Hernández, P.; Rojas, R.; Gilman, R. H.; Sauvain, M.; Lima, L. M.; Barreiro, E. J.; Cerecetto, H. European journal of medicinal chemistry. 2013, 59, 64-74.
CrossRef - Magda, A. A.; Abdel-Aziz, N. I.; Alaa, A. M.; El-Azab, A. S.; ElTahir, K. E. Bioorganic & medicinal chemistry. 2012, 20, 3306-3316.
CrossRef - Chovatia, P. T.; Akabari, J. D.; Kachhadia, P. K.; Zalavadia, P. D.; Joshi, H. S. Journal of the Serbian Chemical Society. 2006, 71, 713-720.
CrossRef - Chimenti, F.; Bolasco, A.; Manna, F.; Secci, D.; Chimenti, P.; Befani, O.; La Torre, F. Journal of medicinal chemistry. 2004, 47, 2071-2074.
CrossRef - Christodoulou, M. S.; Liekens, S.; Kasiotis, K. M.; Haroutounian, S. A. Bioorganic & medicinal chemistry. 2010, 18, 4338-4350.
CrossRef - Renuka, N.; Kumar, K. A. Bioorganic & medicinal chemistry letters. 2013, 23, 6406-6409.
CrossRef - Akbas, E.; Berber, I.; Sener, A.; Hasanov, B. Il Farmaco. 2005, 60, 23-26.
CrossRef - Zhang, D.; Wang, G.; Zhao, G.; Xu, W.; Huo, L. European journal of medicinal chemistry. 2011, 46, 5868-5877.
CrossRef - Baciu-Atudosie, L.; Ghinet, A.; Farce, A.; Dubois, J.; Belei, D.; Bicu, E. Bioorganic & medicinal chemistry letters. 2012, 22, 6896-6902.
CrossRef - Bai, X. G.; Yu, D. K.; Wang, J. X.; Zhang, H.; He, H. W.; Shao, R. G.; Wang, Y. C. Bioorganic & medicinal chemistry letters. 2012, 22, 6947-6951.
CrossRef - Bavetsias, V.; Crumpler, S.; Sun, C.; Avery, S.; Atrash, B.; Faisal, A.; Blagg, J. Journal of medicinal chemistry. 2012, 55, 8721-8734.
CrossRef - Mishra, S. K.; Sahoo, S.; Panda, P. K.; Mishra, S. R.; Mohanta, R. K.; Ellaiah, P.; Panda, C. S. Acta poloniae pharmaceutica. 2007, 64, 359-364.
- Mu, J. X.; Shi, Y. X.; Yang, M. Y.; Sun, Z. H.; Liu, X. H.; Li, B. J.; Sun, N. B. Molecules. 2016, 21, 68.
CrossRef - Argade, N. D.; Kalrale, B. K.; Gill, C. H. E-Journal of chemistry. 2008, 5, 120-129.
CrossRef - Karthikeyan, M. S.; Holla, B. S.; Kumari, N. S. European journal of medicinal chemistry. 2007, 42, 30-36.
CrossRef - Ragavan, R. V.; Vijayakumar, V.; Kumari, N. S. European journal of medicinal chemistry. 2010, 45, 1173-1180.
CrossRef - Menozzi, G.; Merello, L.; Fossa, P.; Schenone, S.; Ranise, A.; Mosti, L.; Tamburini, E. Bioorganic & medicinal chemistry. 2004, 12, 5465-5483.
CrossRef - Gokhan-Kelekci, N.; Yabanoglu, S.; Kupeli, E.; Salgın, U.; Ozgen, O.; Uçar, G.; Bilgin, A. A. Bioorganic & medicinal chemistry. 2007, 15, 5775-5786.
CrossRef - Manojkumar, P.; Ravi, T. K.; GOPALAKRISHNAN, G. Acta Pharmaceutica. 2009, 59, 159-168.
CrossRef - Rao Jyothi, N.; Sujith, K. V.; Kalluraya, B. Saudi Chem. Soc. 2008, 12, 347-52.
CrossRef - Sahu, S. K.; Banerjee, M.; Samantray, A.; Behera, C.; Azam, M. A. Tropical journal of pharmaceutical research. 2008, 7, 961-968.
CrossRef - Al-Adiwish, W. M.; Tahir, M. I. M.; Siti-Noor-Adnalizawati, A.; Hashim, S. F.; Ibrahim, N.; Yaacob, W. A. European journal of medicinal chemistry. 2013, 64, 464-476.
CrossRef - Bavetsias, V.; Faisal, A.; Crumpler, S.; Brown, N.; Kosmopoulou, M.; Joshi, A.; Blagg, J. Journal of medicinal chemistry. 2013, 56, 9122-9135.
CrossRef - Shen, S. L.; Shao, J. H.; Luo, J. Z.; Liu, J. T.; Miao, J. Y.; Zhao, B. X. European journal of medicinal chemistry. 2013, 63, 256-268.
CrossRef - Ali, A. R.; El-Bendary, E. R.; Ghaly, M. A.; Shehata, I. A. European journal of medicinal chemistry. 2014, 75, 492-500.
CrossRef - Sun, J.; Lv, X. H.; Qiu, H. Y.; Wang, Y. T.; Du, Q. R.; Li, D. D.; Zhu, H. L. European journal of medicinal chemistry. 2013, 68, 1-9.
CrossRef - Park, B. S.; Al-Sanea, M. M.; Abdelazem, A. Z.; Park, H. M.; Roh, E. J.; Park, H. M.; Lee, S. H. Bioorganic & medicinal chemistry. 2014, 22, 3871-3878.
CrossRef - Reddy, G. L.; Guru, S. K.; Srinivas, M.; Pathania, A. S.; Mahajan, P.; Nargotra, A.; Sawant, S. D. European journal of medicinal chemistry. 2014, 80, 201-208.
CrossRef - Wang, S. F.; Zhu, Y. L.; Zhu, P. T.; Makawana, J. A.; Zhang, Y. L.; Zhao, M. Y.; Zhu, H. L. Bioorganic & medicinal chemistry. 2014, 22, 6201-6208.
- Yao, Y.; Liao, C.; Li, Z.; Wang, Z.; Sun, Q.; Liu, C.; Jiang, S. European journal of medicinal chemistry. 2014, 86, 639-652.
- Kamal, A.; Shaik, A. B.; Polepalli, S.; Kumar, G. B.; Reddy, V. S.; Mahesh, R.; Jain, N. Bioorganic & medicinal chemistry. 2015, 23, 1082-1095.
- Khloya, P.; Ceruso, M.; Ram, S.; Supuran, C. T.; Sharma, P. K. Bioorganic & medicinal chemistry letters. 2015, 25, 3208-3212.
- Maggio, B.; Raimondi, M. V.; Raffa, D.; Plescia, F.; Cascioferro, S.; Cancemi, G.; Daidone, G. European journal of medicinal chemistry. 2015, 96, 98-104.
- Nitulescu, G. M.; Draghici, C.; Olaru, O. T.; Matei, L.; Ioana, A.; Dragu, L. D.; Bleotu, C. Bioorganic & medicinal chemistry. 2015, 23, 5799-5808.
- Nitulescu, G. M.; Matei, L.; Aldea, I. M.; Draghici, C.; Olaru, O. T.; Bleotu, C. Arabian Journal of Chemistry. 2019, 12, 816-824.
- Rai, U. S.; Isloor, A. M.; Shetty, P.; Pai, K. S. R.; Fun, H. K. Arabian Journal of Chemistry. 2015, 8, 317-321.
- Reddy, T. S.; Kulhari, H.; Reddy, V. G.; Bansal, V.; Kamal, A.; Shukla, R. European journal of medicinal chemistry. 2015, 101, 790-805.
- Shi, J. B.; Tang, W. J.; Li, R.; Liu, X. H. European journal of medicinal chemistry. 2015, 90, 889-896.
- Huang, Q. P.; Zhang, S. N.; Zhang, S. H.; Wang, K.; Xiao, Y. Molecules. 2017, 22, 1813.
- Daidone, G.; Maggio, B.; Raffa, D.; Plescia, S.; Schillaci, D.; Raimondi, M. V. Il Farmaco. 2004, 59, 413-417.
- Qiu, K. M.; Yan, R.; Xing, M.; Wang, H. H.; Cui, H. E.; Gong, H. B.; Zhu, H. L. Bioorganic & medicinal chemistry. 2012, 20, 6648-6654.
- Alegaon, S. G.; Alagawadi, K. R.; Garg, M. K.; Dushyant, K.; Vinod, D. Bioorganic chemistry. 2014, 54, 51-59.
- Karrouchi, K.; Chemlal, L.; Taoufik, J.; Cherrah, Y.; Radi, S.; Faouzi, M. E. A.; Ansar, M. Annales pharmaceutiques francaises. 2016, 74, 431-438.
- Selvam, T. P.; Kumar, P. V.; Saravanan, G.; Prakash, C. R. Journal of Saudi Chemical Society. 2014, 18, 1015-1021.
- Tewari, A. K.; Singh, V. P.; Yadav, P.; Gupta, G.; Singh, A.; Goel, R. K.; Mohan, C. G. Bioorganic chemistry. 2014, 56, 8-15.
- El-Feky, S. A.; Abd El-Samii, Z. K.; Osman, N. A.; Lashine, J.; Kamel, M. A.; Thabet, H. K. Bioorganic chemistry. 2015, 58, 104-116.
- Hussain, S.; Kaushik, D. Journal of Saudi Chemical Society. 2015, 19, 274-281.
- Kumar, R. S.; Arif, I. A.; Ahamed, A.; Idhayadhulla, A. Saudi journal of biological sciences. 2016, 23, 614-620.
- Li, Y. R.; Li, C.; Liu, J. C.; Guo, M.; Zhang, T. Y.; Sun, L. P.; Piao, H. R. Bioorganic & medicinal chemistry letters. 2015, 25, 5052-5057.
- Pelcman, B.; Sanin, A.; Nilsson, P.; Schaal, W.; Olofsson, K.; Krog-Jensen, C.; Claesson, H. E. Bioorganic & medicinal chemistry letters. 2015, 25, 3017-3023.
- Thore, S. N.; Gupta, S. V.; Baheti, K. G. Journal of Saudi Chemical Society. 2016, 20, 259-264. .
- de Moura, S. S.; de Ávila, R. I.; Brito, L. B.; de Oliveira, R.; de Oliveira, G. A. R.; Pazini, F.; Valadares, M. C. Chemico-biological interactions. 2017, 277, 185-194.
- Ragab, F. A.; Gawad, N. M. A.; Georgey, H. H.; Said, M. F. European journal of medicinal chemistry. 2013, 63, 645-654.
- Viveka, S.; Shama, P.; Nagaraja, G. K.; Ballav, S.; Kerkar, S. European journal of medicinal chemistry. 2015, 101, 442-451.
- Thore, S. N.; Gupta, S. V.; Baheti, K. G. Journal of Saudi Chemical Society. 2016, 20, S46-S52.
- Chowdhury, M. A.; Abdellatif, K. R.; Dong, Y.; Knaus, E. E. Bioorganic & medicinal chemistry. 2008, 16, 8882-8888.
- Chowdhury, M. A.; Abdellatif, K. R.; Dong, Y.; Yu, G.; Huang, Z.; Rahman, M.; Knaus, E. E. Bioorganic & medicinal chemistry letters. 2010, 20, 1324-1329.
- Singh, S. K.; Reddy, P. G.; Rao, K. S.; Lohray, B. B.; Misra, P.; Rajjak, S. A.; Venkateswarlu, A. Bioorganic & medicinal chemistry letters. 2004, 14, 499-504.
- Li, J.; DeMello, K. M. L.; Cheng, H.; Sakya, S. M.; Bronk, B. S.; Rafka, R. J.; Silvia, A. Bioorganic & medicinal chemistry letters. 2004, 14, 95-98.
- Cheng, H.; DeMello, K. M. L.; Li, J.; Sakya, S. M.; Ando, K.; Kawamura, K.; Seibel, S. B. Bioorganic & medicinal chemistry letters. 2006, 16, 2076-2080.
- Sakya, S. M.; DeMello, K. M. L.; Minich, M. L.; Rast, B.; Shavnya, A.; Rafka, R. J.; Lynch, M. P. Bioorganic & medicinal chemistry letters. 2006,16, 288-292.

This work is licensed under a Creative Commons Attribution 4.0 International License.









