Analytical Instrumentation Techniques of FT-IR, XRD, SEM, and EDX for Adsorption Methods of Ni2+ Ions Onto Low Cost Adsorbent
Department of Chemistry, Poompuhar College(AU),(Affiliated to Bharathidasan University),Melaiyur-609107, Tamilnadu, India.
Corresponding Author E-mail: arivu6363@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370608
Article Received on : 22-Oct-2021
Article Accepted on :
Article Published : 07 Dec 2021
Reviewed by: Dr. Soon Min Ho

Second Review by: Dr. Athanasius P Bayuseno

Final Approval by: Dr. Vandana
The present study investigates the possible removal of Ni2+ ions from aqueous solution by using low-cost Hygrophila auriculata activated nano carbon (HA-ANC) as an adsorbent. The activated nano carbon had been prepared from Hygrophila auriculata stem waste as well; the raw material was carbonized with con. H2SO4 and activated by thermal action. Batch experiments were performed in order to calculate the percentage removal of Ni2+ ions for 90.737% at 60 oC. The properties of treated carbon and untreated carbon are compared using instrumental techniques such as FT-IR, XRD, SEM and EDX, which confirms Ni2+ ions adsorption onto HA-ANC. FT-IR showed that the surface of HA-ANC had more oxygen containing functional groups which enhanced the adsorption of Ni2+. XRD showed the nature of adsorbent, SEM images implies morphological deviance of before and after adsorption of Ni2+ onto HA-ANC and EDX showed that the C content of HA-ANC were higher than that of Ni2+/ HA-ANC.
KEYWORDS:Adsorption; Batch method, Ni2+ ions; EDX; FT-IR; Hygrophila Auriculata Activated Nano Carbon (HA-ANC); SEM; XRD
Download this article as:| Copy the following to cite this article: Arivoli S, Ajithkumar M. Analytical Instrumentation Techniques of FT-IR, XRD, SEM, and EDX for Adsorption Methods of Ni2+ Ions Onto Low Cost Adsorbent. Orient J Chem 2021;37(6). |
| Copy the following to cite this URL: Arivoli S, Ajithkumar M. Analytical Instrumentation Techniques of FT-IR, XRD, SEM, and EDX for Adsorption Methods of Ni2+ Ions Onto Low Cost Adsorbent. Orient J Chem 2021;37(6).Available from: https://bit.ly/31uC0z0 |
Introduction
Pollution of heavy metals has attracted global attention due to their toxicity, hard decay and accumulation in organisms. Heavy metals accumulate in the tissues of various living organisms and as a result attach to the food chain, affecting humans, posing a health risk 1-3. Therefore, the treatment of wastewater polluted by heavy metals is an important environmental concern. Ion exchange, solvent extraction, chemical precipitation, ultra-filtration, reverse osmosis, electro dialysis and adsorption are the traditional methods for removing heavy metal ions from polluted water 4-6. However, absorption is considered to be one of the most popular methods for removing heavy metals from wastewater due to its low cost, in effect, biodegradability, ease of design and high removal efficiency. There are many absorbent materials available, but activated carbon is used to remove heavy metals by the adsorption method because activated carbon has a numerous pores and a large surface area 7, 8.
The literary study shows that no work has been done on Hygrophila auriculata activated nano carbon (HA-ANC) as an adsorbent, as well as low cost and high abundance. Therefore this study focused on the removal of Ni2+ ions from the aqueous solution using the HA-ANC by batch adsorption method.
Materials and Methods
Chemicals
Analytical reagent grade chemicals were used. Stock Ni2+ ion solution (1000 ppm) was prepared by dissolving required amount of NiSO4.6H2O in 1000 ml of double distilled water. Working standards were prepared by diluting the stock solution of Ni2+ ions using double distilled water. The ionic solutions of 0.1M HCl and 0.1MNaOH were made for to alter the solution pH.
Procedure for Adsorbent Preparation and Activation
The Hygrophila auriculata stem waste was obtained from the agricultural sites near at Poompuhar, Mayiladuthurai district, Tamilnadu, India. The stem was cut into small pieces, dried in the absence of sunlight and treated with concentrated H2SO4 in W/V ratio. The carbonized material washed away by double distilled water until it becomes neutral one and dried further inside the hot air oven at 110 oC for 24 hrs. The carbon was activated by using muffle furnace at 1000 oC for 6 hours and the activated carbon (HA-ANC) was powdered well and stored in desiccators in order to perform the experiment 9.
Batch Adsorption Method
The batch experiments [10] for Ni2+ ions removal was determined under various initial pH like 3, 4, 5, 6, 7, 8 and 9 besides temperature ranges from 30 to 60 oC at an initial Ni2+ ion solution concentration was varied from 10 to 50 ppm with different adsorbent doses and different time intervals such as 15, 30, 45, and 60 minutes. The effect of initial Ni2+ ions concentrations was investigated by using 25 mg biomass, initial pH 6 and volume of Ni2+ ion solution 50 mL are constant but varied initial Ni2+ concentrations at room temperature (30 oC). The Ni2+ ion solution samples were collected after 60 min. of shaking then centrifuged at 120 rpm, the filtrate was analyzed. The unadsorbed Ni2+ ions were measured by using UV-Visible spectrophotometer. The removal percentage and amount of Ni2+ ions adsorption were calculated using equation 1 and equation 2 correspondingly.
Mass Balance Relationship Equation Eq. No.
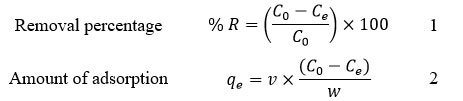
Where, % R is the percentage of removal, qe is the amount of adsorbed Ni2+ ions per unit quantity of adsorbent at equilibrium time (mg g -1), C0 and Ce are the initial and actual concentration (ppm) of Ni2+ ion solutions at equilibrium time, respectively. The V is the volume of the Ni2+ ion solution (L), ‘w’ is the weight of the HA-ANC (g).
Analytical Tools for HA-ANC and Ni2+/HA-ANC
The determination of surface area, volume and diameter of pores for HA-ANC, BET-BJH methods were used. The FT-IR spectrum is an important tool to study the changes infrequency due to the interaction between adsorbent and adsorbate. The XRD pattern confirms the nature of the adsorbent as well as adsorption of Ni2+ ions onto HA-ANC in order to changes of 2θ values. SEM is a substantial tool to evaluate the morphological features and surface characteristics of the fresh adsorbent and treated adsorbent materials and also EDX study gives details about the element compositions 11, 12.
Results and Discussions
The equilibrium data was calculated with the help of batch methodology it’s incorporated some of the effective parameters such as contact time, solution pH, adsorbent dose, temperature 13. The data was given in table 1, the result says that the nature of effectiveness of Hygrophila auriculata activated nano carbon (HA-ANC), which indicates the amount of adsorbed Ni2+ ions onto HA-ANC increased with increasing temperature. The removal was high (90.737%) at 60 oC it’s also declared the removal of Ni2+ ions onto adsorbent favored in elevation of temperature as well as low concentration of Ni2+ ion solution by 25 mg of adsorbent.
Table 1: Equilibrium Data for the Adsorption of Ni2+ ions onto HA-ANC
|
C0 |
Ce (Mg / L) |
qe (Mg / L) |
Removal % |
|||||||||
|
30oC |
40oC |
50oC |
60oC |
30oC |
40oC |
50oC |
60oC |
30oC |
40oC |
50oC |
60oC |
|
|
10 |
1.804 |
1.463 |
0.976 |
0.926 |
16.393 |
17.075 |
18.048 |
18.147 |
81.963 |
85.375 |
90.238 |
90.737 |
|
20 |
5.363 |
4.877 |
4.408 |
3.414 |
29.275 |
30.246 |
31.184 |
33.173 |
73.188 |
75.614 |
77.960 |
82.932 |
|
30 |
11.149 |
10.630 |
9.263 |
8.582 |
37.702 |
38.741 |
41.475 |
42.836 |
62.836 |
64.568 |
69.125 |
71.393 |
|
40 |
17.103 |
15.641 |
14.837 |
13.917 |
45.793 |
48.718 |
50.327 |
52.166 |
57.241 |
60.897 |
62.908 |
65.208 |
|
50 |
25.350 |
24.471 |
23.931 |
22.510 |
49.300 |
51.059 |
52.139 |
54.981 |
49.300 |
51.059 |
52.139 |
54.981 |
According to the BET-BJH method, the surface area, volume and diameter of pores was obtained as 90.067 m²/g, 0.115 cc/g and 3.520 nm, respectively.
Fourier transform infrared (FT-IR) was used to determine the changes of vibration frequency in the functional groups of the adsorbent due to Ni2+ ions adsorption. The FT-IR spectrum within 500–4000 cm-1 for the HA-ANC before and after the adsorption of Ni2+ is shown in order to fig. 1, and fig. 2. The peak point of frequency; 2500 to 4000 cm-1 indicates the single bonds (C-H, O-H, N-H), 2000 to 2500 cm-1 indicates the triple bonds (C≡C, C≡N), 1500 to 2000 cm-1 indicates the double bonds (C=C, C=N, C=O) and 500 to 1500 cm-1 indicates fingerprint region. The FT-IR spectrum of HA-ANC indicates that there is remarkable change in the peaks at 2997.49 cm-1, 2888.47 cm-1, 1860.25 cm-1, 1560.72 cm-1, 1184.19 cm-1, 1123.42 cm-1, 1035.85 cm-1, 795.09 cm-1, and 525.96 cm-1 respectively which it can be due to Ni2+ binding with functional groups adsorbent. The formation of new peaks, demolition of old peaks and higher to lower as well as lower to higher shifting of peaks were reason for that HA-ANC adsorbed with Ni2+ions 14-16.
 |
Figure 1: FT-IR for HA-ANC |
 |
Figure 2: FT-IR for Ni2+/HA-ANC |
The result of XRD diffractogram for HA-ANC (fig. 3) revealed that the Hygrophila auriculata activated nano carbon is crystalline in nature and resembles the graphite structure 17-19. After adsorption of Ni2+ ions (fig. 4) the surface of adsorbent was disturbed this one leads to new theta values, HA-ANC given in table 2 and Ni2+/HA-ANC given in table 3 respectively.
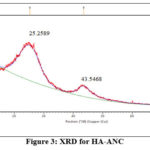 |
Figure 3: XRD for HA-ANC |
 |
Figure 4: XRD for Ni2+/ HA-ANC |
Table 2: XRD measurements of HA-ANC
|
Pos. [°2θ] |
Height [cts] |
FWHM Left [°2θ] |
d-spacing [Å] |
Rel. Int. [%] |
|
25.2589 |
1233.20 |
8.7438 |
3.52305 |
100.00 |
|
43.5468 |
384.92 |
4.5991 |
2.07663 |
31.21 |
Table 3: XRD measurements of Ni2+/ HA-ANC
|
Pos. [°2θ] |
Height [cts] |
FWHM Left [°2θ] |
d-spacing [Å] |
Rel. Int. [%] |
|
5.0131 |
23772.90 |
0.0520 |
17.61333 |
100.00 |
|
20.3762 |
708.42 |
3.3002 |
4.35492 |
2.98 |
|
23.8854 |
1206.41 |
5.9034 |
3.72244 |
5.07 |
|
26.5138 |
209.24 |
9.0362 |
3.35909 |
0.88 |
|
42.5192 |
718.56 |
0.9656 |
2.12441 |
3.02 |
SEM images have been used for morphological study of HA-ANC. The SEM micrographs of HA-ANC before and after adsorption of Ni2+ ions are shown in Fig. 5,and Fig.6, respectively. In the SEM image of HA-ANC, the porous structure is obvious, but in the case of the used particles, the pores have been covered by the Ni2+ions 20, 21.
 |
Figure 5: SEM image for HA-ANC in different magnifications 10 µm, 1 µm and 300 nm |
 |
Figure 6: SEM image for Ni2+/ HA-ANC in different magnifications 10 µm, 1 µm and 300 nm |
The EDX spectrum 22, 23 of HA-ANC before and after adsorption of Ni2+ ions were evaluated shown in fig. 7 and fig. 8 respectively. The spectrum clearly indicates the peak of element C, there was significant reduction in the intensity of peak of C in the after adsorption of Ni2+ ions comparing with the before adsorption. And also the peak of Ni2+ ions was notable on the after adsorption of Ni2+ ions onto HA-ANC in addition the elemental compositions statistics were given in Table 4 and Table 5 respectively for before and after adsorption Ni2+ ions onto HA-ANC. In addition the atomic percentage of carbon reduced form 99.7% to 88.8% bassed on Table 4 and Table 5, it can be usable as remarkable reduction.
 |
Figure 7: EDX for HA-ANC |
 |
Figure 8: EDX for Ni2+/HA-ANC |
Table 4: EDX of Smart Quant Results for HA-ANC
|
Element |
Weight % |
Atomic % |
Error % |
Kratio |
|
C K |
85.6 |
89.7 |
4.4 |
0.5959 |
|
O K |
11.7 |
9.2 |
13.9 |
0.0133 |
|
MgK |
0.5 |
0.2 |
13.2 |
0.0029 |
|
AlK |
0.4 |
0.2 |
13.2 |
0.0026 |
|
SiK |
0.7 |
0.3 |
7.2 |
0.0056 |
|
S K |
0.3 |
0.1 |
18.3 |
0.0028 |
|
ClK |
0.4 |
0.1 |
19.9 |
0.0035 |
|
CaK |
0.4 |
0.1 |
27.7 |
0.0034 |
Table 5: EDX of Smart Quant Results for Ni2+/HA-ANC
|
Element |
Weight % |
Atomic % |
Error % |
Kratio |
|
C K |
84.8 |
88.8 |
4.0 |
0.6254 |
|
O K |
13.5 |
10.6 |
13.6 |
0.0158 |
|
AlK |
0.4 |
0.2 |
9.9 |
0.0031 |
|
S K |
0.4 |
0.2 |
15.4 |
0.0038 |
|
ClK |
0.2 |
0.1 |
30.2 |
0.0020 |
|
NiK |
0.6 |
0.1 |
20.0 |
0.0062 |
Conclusion
The Hygrophila auriculata activated nano carbon (HA-ANC)has been used successfully as an adsorbent for the removal of Ni2+ ions. The removal of Ni2+ ions was found to be 90.737% with an initial concentration of 10 ppm at pH 6 in 60 minutes. The appearance, disappearance and shifting of peaks in the FT-IR spectrum confirmed the adsorption of Ni2+ ions onto HA-ANC as well as XRD analysis also provided reasonable evidence for adsorption to the adsorbent. In addition to supporting, the SEM study gives difference in the surface morphology of the adsorbent before and after the adsorption of Ni2+ ions as well as EDX also given elemental composition evident of removal of Ni2+ ions onto HA-ANC. Finally, it was concluded that the present adsorbent HA-ANC could be a good alternative adsorbent for the removal of heavy metals from aqueous solutions in a very efficient and economical manner.
Acknowledgement
The authors sincerely thank the Government of Tamil Nadu Adi Dravidar and Tribal Welfare Department for providing the fund from Full Time Ph.D Scholars incentive Scheme.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Mousa WM, Soliman SI, El-Bialy AB, Hanaa A Shier. Journal of Applied Sciences Research, 2013; 9(3):1696-170.
- Pandharipande SL, Rohit P, Kalnake P. International Journal of Engineering Sciences & Emerging Technologies, 2013; 4(2):83-89.
- Babel S, Kurniawan TA. Journal of Hazardous Materials, 2003; 97:219-243.
CrossRef - Figoli A, Cassano A, Criscuoli A, Mozumder MSI, Uddin MT, Islam MA, Driolo E. Water Research, 2010; 44:97-104.
CrossRef - Arivoli S, Venkatraman BR, Rajachandrasekar T, Hema M. Research Journal of Chemistry and Environment, 2007; 17:70-78.
- Arivoli S, Kalpana K, Sudha R, Rajachandrasekar T. E-Journal of Chemistry, 2007; 4:238-254.
CrossRef - Coogan TP, Latta DM, Snow ET, Costa M, Lawrence A. Critical Reviews in Toxicology, 1989; 19:341-384.
CrossRef - Savolainen H. Reviews on environmental health, 1996; 11:167-173.
CrossRef - Arivoli S, Hema M, Parthasarathy S, Manju N. Journal of Chemical and Pharmaceutical Research, 2010; 2(5):626-641.
- Arivoli S, Hema M. International Journal of Physical Sciences, 2007; 2:10-17.
- Nhamo Chaukura, Edna C. Murimba, Willis Gwenzi, Applied Water Science, 2017; 7:2175–2186
CrossRef - Chantakorn Patawat, Ketsara Silakate, Somchai Chuan-Udom, Nontipa Supanchaiyamat, Andrew J. Hunt, Yuvarat Ngernyen, RSC Advances, 2020; 10:21082–21091.
- Arivoli S, Viji Jain M, Rajachandrasekar T. Material Science Research India, 2006; 3:241-250.
CrossRef - El-Sadaawy M, Abdelwahab O, Alexandria Engineering Journal, 2014; 53(2):399–408.
CrossRef - Gonzalez JF, Silvia R, Gonzalez G, Carmen M, Nabais JM, Valente, Luis OA. Industrial & Engineering Chemistry Research, 2009; 48:7474-7481.
CrossRef - Muniandy L, Adam F, Mohamed AR, E-P Ng. Microporous and Mesoporous Materials, 2014; 197:316–323.
CrossRef - Pavan Kumar GVSR, Komal Avinash Malla, Bharath Yerra, Srinivasa Rao K, Applied Water Science, 2019; 9(3):44,1-9.
CrossRef - Qu D. Journal of Power Sources, 2002; 109:403-411.
CrossRef - Bratek W, Świa̧ tkowski A, Pakuła M, Biniak S, Bystrzejewski, Szmigielski R, Journal of Analytical and Applied Pyrolysis, 2013; 100:192-198.
CrossRef - El-Araby H.A, Ibrahim AMMA, Mangood AH, Abdel-Rahman AAH, Journal of Geoscience and Environment Protection, 2017; 5:109-152.
CrossRef - Gil R R, Ruiz B, Lozano MS, Fuente E, Journal of Analytical and Applied Pyrolysis, 2014; 110(1): 194–204.
CrossRef - Agegnehu Alemu, Brook Lemma, Nigus Gabbiye, Melisew Tadele Alula, Minyahl Teferi Desta. Heliyon, 2018; 4(e00682):1-22.
CrossRef - Xie Youping, Li Hang, Wang Xiaowei, Ng I-Son, Lu Yinghua, Jing Keju. Journal of the Taiwan Institute of Chemical Engineers, 2014; 45(4):1773–1782.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









