Preparation and Characterization Studies of Amorphophalluspaeonifolius and Manihotesculenta as a Bio-Plastic using Glycerol and Agar-Agar as Plasticizer
J. Morris Princey* , A. Nandhini
, A. Nandhini and E. Abinaya
and E. Abinaya
Department of Chemistry, Holy Cross College (Autonomous), Tiruchirapalli-620002, Tamil Nadu, India.
Corresponding Author E-mail: princeymorris@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360627
Bio-plastics are biodegradable materials which can be obtained from the renewable sources such as corn starch, straw, vegetable fats and oils, wood-chips, recycled food waste, saw dust etc. They can be used as an alternative to the commercial plastics in the market which contaminate our environment. Now-a-days bio-plastics are worldwide popular due to its concern towards the environment, limited fossil fuel resources, and the climatic change. The purpose of this study is to assess the properties of the starch based bio-plastics prepared from Amorphophalluspaeonifolius andManihotesculenta usingglycerol and agar-agar as the plasticizers. The synthesized bio-plastic was characterized with the help of UV-Visible spectrophotometer, FT-IR spectroscopy and SEM Analysis.
KEYWORDS:Amorphophalluspaeonifolius: FT-IR and UVspectroscopic Studies; Manihotesculenta: Physico-chemical parameters; SEM analysis; Starch isolation
Download this article as:| Copy the following to cite this article: Princey J. M, Nandhini A, Abinaya E. Preparation and Characterization Studies of Amorphophalluspaeonifolius and Manihotesculenta as a Bio-Plastic using Glycerol and Agar-Agar as Plasticizer. Orient J Chem 2020;36(6). |
| Copy the following to cite this URL: Princey J. M, Nandhini A, Abinaya E. Preparation and Characterization Studies of Amorphophalluspaeonifolius and Manihotesculenta as a Bio-Plastic using Glycerol and Agar-Agar as Plasticizer. Orient J Chem 2020;36(6). Available from: https://bit.ly/2JNIJLp |
Introduction
The environmental impact of plastic wastes is one of the current concerns due to the accumulation of the non-biodegradable plastics on earth. Plastics play a vital role in packaging industry and in the recent years over 67 million tons1 of packaging waste results in a negative environmental impact. Therefore the synthesis of bio-polymers has generated a great interest amongst the researchers as they bring a significant contribution to the sustainable development with a wider range of disposal options.Bio-plastics are biodegradable plastics, derived from biological substances like corn and sugarcane rather than petroleum.According to the European Bio-plastics Organization, bio-plastics are defined as “Plastics based on renewable or bio-based resources or plastics which are biodegradable or compostable”. They are 100% degradable, resistant and versatile and they are used to develop medical materials used in packaging, cosmetics, food additives and clothing. A polymer categorized to be bio-plastic must be bio-degradable, it should not be a potential threat to the growth of the plants and it must disintegrate2 within a time frame of two months. Bio-plastics are water insoluble, resistant, optically pure and impermeable to oxygen. Many scientists work on biodegradable polymers as a substitute for petrochemical based polymers, focusing on environmental, economic and safety challenges. These bio-degradable polymers or bio-plastics show considerable eco-friendly surroundings than the conventional plastics. Cassava and elephant foot yam are important food3 and cash crops in south Asia. The present study aims in the preparation and characterization of bio-plastics from cassava (Manihotesculenta) and elephant foot yam starch (Amorphophalluspaeonifolius) using glycerol and agar- agar as plasticizers. Glycerol is widely used as a plasticizer4 in fabricating the bioplastics. The bio-plastics obtained were further studied for their physico-chemical5 and spectroscopic properties.
Materials and Methods
Starch Extraction
The fresh tubers were peeled off and washed carefully in water to remove soil and other particles. After the manual peeling it was cutintosmall pieces and dried. After drying, the tuber was grinded into a fine powder after which the powder was submerged in distilled water for about24 hours. After 24 hours, filtration was done using a muslin cloth. The starch was dewatered and dried at sunlight. Finally, the dried starch was collected in container and used for further studies6.
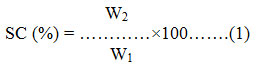
where, SC is Starch isolation, W2 is dry weight of the sample and W1 is wet weight of the sample.
Test for Moisture Content of Starch
The petri dishes with lids were washed and dried in an oven at 105◦C and cooled to room temperature7 in a desiccator. Approximately 2g of starch samples were weighed accurately in the petri dishes. The samples were dried for 8 hours at 120◦C, cooled in desiccator and weighed.The moisture content was calculated using equation 2
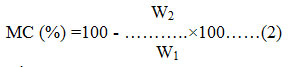
where, MC is Moisture content, W2 is dry weight of the sample, W1 is wet weight of the sample.
Test for Ash Content
The crucibles were cleaned, heated for 30 minutes at 200◦C, cooled to room temperature in adesiccator. Approximately 2g of the starch sample (W1) were weighed accurately in the crucible and incinerated on a Bunsen burner until the carbonization of the sample was complete. Then the incineration was done at 200◦Cfor about2hours. The incinerated samples were cooled in a desiccator to room temperature and weighed (W2)
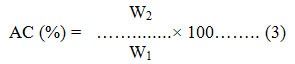
where, AC is the Ash Content, W2 is dry weight and W1 is wet Weight
pH of the Starch
About 20% W/V dispersion of the sample was shaken in water for 5 min,the starch was allowed to settle and the pH of the water phase was determined using a calibrated pH meter8.
Titratable Acidity
2g of the starch sample was suspended in 20 ml distilled water and titrated against 0.1 M NaOHusing phenolphthalein as the indicator.
Amylose Content
About 0.1g of the starch sample was taken ina test tube along with 1 ml of 95% ethanol and 9ml of 0.1 M NaOH. The test tube was covered with an aluminum foil and thoroughly mixed and heated for 10 min in a water bath to gelatinize the starch. The suspension was cooled and then dilutedto 10 times its volume. About 0.5 ml of the extract was used in the analysis to which 0.1 ml acetic acid followed by 0.2 ml of iodine in CCl4was added . The solution was made up to upto10 ml with water and kept for 20 minutes for the color development. The absorbance of the solution was read at 620nm9.The amylose content determination was carried out using a colorimetric iodine affinity procedure.
Bio-plastic Preparation
The bio-plastics were made using the starch of cassava and elephant foot yam. About 2g of the cassava starch was taken in a beaker with 10 ml of the distilled water and stirred well to which 1.5 ml acetic acid and 0.5 ml glycerol10 was added. The mixture was heated in a Bunsen burner with continuous stirring until the formation of a white colloidal gel. The gel was poured as a flat sheet dried at room temperature for about 48 hours which later developed as a white film.The bio-plastic from elepahant foot yam was also developed in the similar method using agar –agar and glycerol as the plasticizers.
Water Absorption Capacity
The samples were dried in the oven for about 24 hours at 50℃, cooled in a desiccator and weighed. The water absorption test was done for one hour of immersion after which the samples were reweighed11. The water uptake capacity of the film was calculated using Equation 4.
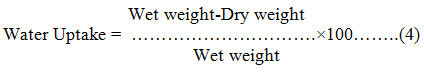
Biodegradability Test
The biodegradable plastics produced were cut into 1×1 cm and buried in a small rectangular hole in a compost soil at a depth of 12cm for a period of two weeks. The degradation of the film was monitored at regular intervals. The weight of the samples before (W0) and after burial (Wf) in the compost soil were noted and the weight loss of the samples were calculated12 using Equation 5.
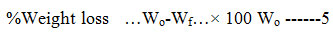
Application of bio-plastics as packaging for liquid
The application test was conducted using groundnut oil and distilled water13 as the test samples. The bio-plastics were cut into 1cm x1cm, initial weight (W1) was taken and immersedwith the test sample in a beaker. The beaker was covered and kept at room temperature for 120 minutes. After the incubation time was complete the sample was cleansed and weighed to obtain the final weight (W2). The percentage weight change of the bio-plasics was calculated as described in equation 6. If the weight change of bio-plastic after being immersed in the test food products is less than 10% (W2/W1), then the bio-plastics can be considered to be compatible with those test foods.
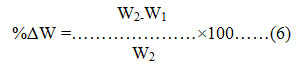
Characterization of the Bio-Plastics
The characterization of the bio-plastics wasdone by different spectroscopic and microscopic techniques .
UV-Visible Spectroscopic Study
The bio-plastic was cut into 1cm× 3cm and the wavelength used was in the range of 300-800nm for the characterization14 of the synthesized bio-plastics.The UV-Vis spectral analysis was done by UV-VIS spectrophotometer.
FT-IR Analysis
The infra-red spectroscopy determines the structure of the molecular components and it is mainly used to find out the functional groups in it. The spectral wavelength range is usually around 4000-400 cm-1. The spectra was obtained by mixing the sample with potassium bromide pellets.
Scanning Electron Microscopy
Scanning electron microscopy is a versatile technique used to collect the information about the topography, morphology,composition and crystallographic information of the sample. In SEM, the electrons are heated at high temperature which emits the electron from the tungstenfilamentand flows towards the anode. The electron beam15used has an energy from the range of few hundred eV to keV.
Results and Discussion
Starch Isolation
The yield of the two starches is shown in Table.1. The yield is appreciable compared with the starches from other sources. Based on the yield, it can be used as a commercial product in packaging industry.
Table 1: Results of physico-chemical properties of Manihotesculentaand Amorphophalluspaeonifolius.
|
No. |
Parameters |
Manihotesculenta |
Amorphophalluspaeonifolius |
|
1. |
Appearance /colour |
White |
Brown |
|
2. |
Odour |
Odourless |
Odourless |
|
3. |
% Yield |
82 |
78.5 |
|
4. |
pH |
5.00 |
7.78 |
|
5. |
Amylose content |
0.5 |
0.22 |
Table 2: Proximate analysis of Manihotesculenta and Amorphophalluspaeonifolius starch.
|
No |
Parameters |
Manihotesculenta |
Amorphophalluspaeonifolius |
|
1. |
Moisture content |
12.18
|
12.16 |
|
2. |
Ash content |
0.11 |
0.109 |
|
3. |
Water uptake |
86 % |
79% |
Moisture Content
The moisture content value of two starches was shown in table in Table.2. The moisture content for the cassava starch and the elephant foot yam was found to be in the range 12-13%16. The moisture contenthelps to maintain the water content in slurry. This test used to determine whether the tuber starch can be used as a packaging material to maintain food longevity. Manihotesculentaand Amorphophalluspaeonifolius starches have higher moisture content compared to other tuber starches. The highmoisture of these starches is a reflection of the loose granules in them, which confirms that the granules are not tightly associated together.
Ash Content
This test helps to determine the typeand the amount of minerals present in the sample.The ash content of both the cassava and the elephant foot yam starches was around 0.11%.The ash content indicates the total minerals in the food17. The mineral and organic salt in the tubers is expressed as the ash content which is the food residue after the combustion process. A maximum ash content of 3% is allowed for the edible tubers.
pH of the Starches
Manihotesculentaand Amorphophalluspaeonifolius were found to have a pH value of 5.0018 and 7.78. The paste clarity depends upon the pH value, due tothe higher pH value, the paste clarity ofthe tuber starches was also high. The value is given in Table 1. Most of the studies
show that starches with the pH range 3-9 can be used in pharmaceutical and food industries.
Amylose Content
The amylose content of starch influences its properties like water binding capacity, thickening, gelling and film forming properties. The high amylose content of the starch will affect its the pasting properties19. Manihotesculentaand Amorphophalluspaeonifolius were found to have a low amylose content value of 0.5% and 0.22%20 which will not affect their film forming property.
Water Absorption Capacity
The water absorbing capacity is very important to determine the water absorptivity of the sample. For the cassava and the elephant foot yam samples it was found to 86% and 79% and the high uptake of water may be due to the presence of three hydroxyl groups in the glycerol21 molecule.The degree of water absorption is also related to its amylose content. If the water holding capacity is high it improves the ability of the starch granules to expand in volume without collapsing.
Biodegradability Test
The biodegradability test was done using soil burial method.The film of the biopolymer was monitored regularly and it was found that after two days micro-organisms were formed on the film after which it started degrading.After a week the film began to change physically where cracks were observed on the surface of the film associated with a weight loss22. This test shows that the bio-plastics synthesized were eco-friendly in nature.
Application of bio-plastic as apackaging materialfor liquids
Table 3: The weight change of the bio-plastics after being immersed in test samples for 2 hours.
|
No. |
Test samples |
Manihotesculenta |
Amorphophalluspaeonifolius |
|
1. |
Distilled water |
87.11 |
55 |
|
2. |
Vegetable oil |
1.26 |
0.97 |
The presence of non- starch components impact the compatibility of bio-plastic food products by delaying the penetration of the vegetable oil into the bio-plastics. In the present study the bio-plastic is made with addition of glycerol. Increased concentration of glycerol in the bio-plastics increase the penetration ofthe vegetable oil to its surface. Here vegetable oil penetration is low because glycerol is used in a minimal volume. Table 3 shows that that the penetration of the vegetable oil is low compared to the penetration of water in the synthesized bio-polymers. This indicates that the bio-plastic of Manihotesculenta and Amorphophalluspaeonifoliuscan be used as a packaging material23 for vegetable oils.
Characterization of Bio-Plastics
UV-Visible Spectroscopy
The UV absorption has significant relationship with the degradation of the plastics. The plastic which does not absorb radiation is not susceptible to photo degradation24.The ability of the bio-plasticsto absorb UV radiation was beneficial when the bio-plastic was used in food packaging. Fig.1 and fig.2 shows the UV spectrogram of the bio-plastics.
 |
Figure 1: shows the UV spectrogram of the bio-plastics. Click here to View figure |
 |
Figure 2: shows the UV spectrogram of the bio-plastics. |
Fig.1,2. UV-Visible spectrum of thedeveloped bio-plastic from Manihotesculenta and Amorphophalluspaeonifolius
Manihotesculenta (cassava) absorbed UV with maximum absorptivity in the wavelength from 350-420 nm25 which is similar to the polycarbonate, one of the common conventional plastics, whereas no such characteristic peak was obtained for Amorphophalluspaeonifolius (elephant foot yam). The bio-plastic obtained from Cassava can protect food products from UV radiation which further prevents their photo-oxidative degradation.
FT-IR Spectroscopy
 |
Figure 3: FT-IR spectrum of developed bio-plastic |
 |
Figure 4: FT-IR spectrum of developed bio-plastic |
Fig.3, 4. FT-IR spectrum of developed bio-plastic from Manihotesculenta andAmorphophalluspaeonifolius
The FT-IR analysis was done to determine the functional groups present in the film. The FT-IR spectrum of the film showed hydrogen bond stretching at 3543.59 cm-1 and the peak at 2928.40cm-1 corresponds to C-H stretching26, the characteristic peak at 1246.09 cm-1 indicates C-O bending of C-O-H group and N=C=N stretching at 2147.58 cm-1 indicated the presence of the cyano group,naturally occurring Manihotesculenta contains cyanide content, when it is raw. If it is cooked, it will be negligible. The FT-IR spectrum of Amorphophalluspaeonifoliusfilm, shows the characteristic peaks at 3596.13cm-1 for the hydroxyl group), 2147.77 cm-1for strong N=C=N stretching , 1669.25cm–1 for C=Cstretching27 (alkene) and 1468.33cm–1for the C-H bending.
Scanning Electron Microscope
 |
Figure 5: SEM micrograph of the developed bio-plastic Click here to View figure |
 |
Figure 6: SEM micrograph of the developed bio-plastic |
Fig.5 and 6. SEM micrograph of the developed bio-plastic Manihotesculenta andAmorphophalluspaeonifolius
SEM analysis of bio-plastic was performed using scanning electron microscope. The SEM micrograph shows the morphology of the bio-plastic and Fig.5 and 6 reveals the homogeneous phase of thedeveloped bio-plastic and spots portion indicates duct particles present on surface. The SEMstudies revealed that Amorphophalluspaeonifolius starchgranulesare roundellipticalin shape with smooth surface.
Conclusion
The future of biodegradable plastics exhibit a great potential since it is eco-friendly, economically viable and it also reduces plastic waste accumulation.In this study starch based bio-plastics were prepared from Manihotesculenta and Amorphophalluspaeonifolius. These starches had high pH, moisture content and high paste clarity than other tuber starches. Bio-plastic from Manihotesculenta can be used as packaging for vegetable oil. These bio-plastics are transparent
enabling to view the product easily. This bio-plastic also protects the product from photo oxidative degradation. The study of Amorphophalluspaeonifolius starch properties contributes to a better understanding of the textural properties of the traditional yam products. The characterization studies and the physico-chemical parameters reveal that these bio-plastics can serve as a good substitute for the conventional plastics which pollute the environment for a long run.
Acknowledgement
The authors are very much thankful to the Principal and Management of Holy Cross College (Autonomous), Tiruchirapalli for the support and encouragement during the work.
Conflict of Interest
The authors declare no conflict of interest.
References
- Satish Kumar; Thakur, K.S. J. Hill Agric., 2017,8(2), 118-129.
CrossRef - Ezeoha, S.L.; Ezenwanne, D.J.N. IOSR J. Eng., 2013, 3(10),14-20.
CrossRef - Parra, D.F.; Tadini, C.C.; Lugao, A.B. Carbohydr. Polym.,2004, 58, 475-481.
CrossRef - Darni,Y. Rasayan J. Chem., 2014, 10, 55-62.
CrossRef - NanouPeelman ; Peter Ragaert ; Bruno De Meulenaer ; DimitriAdons; RoosPeters. Trends in Food Sci Technol., 2013, 32(2), 128-141.
CrossRef - Benesi, I.R.M.; Labuschagne, M.T.;Dixon,A.G.O.; Mahungu, J. Sci. Food Agric., 2004, 84,1381-1388
CrossRef - AshveenNand, V.; RandhirCharan, P; David Rohindra; JajitKhurma, R.; SPJNS, 2008, 26, 45-48
- Ashogbon, A.O., J. Curr. Chem. Phar. Sc., 2014, 4(4), 142-151.
- FagbohunAdebisi ; AfolayanMichaelIkokoh Patrick; OlajideOlutayo.; Adedayo; FatokunOlakunle; AyesanmiAdemola; OrishadipeAbayomi , IJCA,2013, 5(2), 117-126.
- Ana Paula Blick; Carmen Maria Olivera; Juliana BunamettiOlivato; Suzana Mali; Maria Victoria; Fabio Yamashita, Polimeros, 2015, 25, 331-335.
CrossRef - DayangkuIntanMunthoub; Wan Aizan Wan; Abdul Rahman, Sains Malays.,2011, 40(7), 713-718.
- FrancescoPaola La Mantia ;LawaAsicone,Mairia Chiara Mistretta; Marco Repsarde ; Paolo Rizzarelli, Polymers, 2020, 12(40), 753-59.
CrossRef - Obasi, H.C.; Igwe ,I.O., Am. J. Eng. Res.,2011, 2(7),105-114.
- Saamanca , L.E.L.; Cabreta ,L.E.P.; Narveaz ,G.C.D.;Alba ,L,R,B., Proceedings of the 11th International Congress on Engineering and Food; Athena,2011,22-26 May .
- ChagamKoteswara Reddy.; SundaramoorthyHaripriya.; Noor Mohamed.; Suriya,M., Food Chem. ,2015, 155, 38-44.
CrossRef - Surendra Babu, A; Parimalavalli ,R, Int. J. Agric. Food Sci., 2012, 2(3), 77-80.
- RadenCecepErwanAndriansyah; Taufik Rahman; AiniaHerminiati; Nurhaidar Rahman; Rohmah Luthfiyanti, IOP Conf. Ser Earth Environ. Sci., 2017, 101, 1-10.
CrossRef - Thaísa Anders Carvalho Souza; ManoelSoaresJúnior ; Maria Raquel Hidalgo Campos; Thaynara Stella Carvalho Souza; Lívia Cunha Bandeira , Food Sci. Technol, Campinas,2013, 33 (3), 457-462.
CrossRef - Morante , N.; Ceballos, H.; Sa´nchez , T.; Rolland-Sabate, A.; Calle, F.; Hershey, C.; Gibert, O.; Dufour, D., Food Hydrocoll., 2016 , 56(2), 383-395.
CrossRef - ShadrackMubangaChisenga; TilahumSeyoumWorkneh; GermewBurosa; BuliyaminuAdegbemonoAlimi, J. Food Sci. Technol., 2019, 56(6), 2799-2813.
CrossRef - Preechawong ,D.; Peesan,M., Carbohydr. Polym.,2005, 59, 329-337.
CrossRef - Nissa, R.C.; Fikriyyah,A.K.; Abdullah, A.H.D.; Pudjiraharti, S., IOP Conf. Series: Earth and Environmental Science, 2019,277, 1-7.
CrossRef - Ezeoha, S.L.;Ezenwanne , J.N., IOSREN,2013, 3(10), 14-12.
CrossRef - Ribel ,M.J.; US Patent, 2014, 8, 696, 998 B2.
- NoryawatiMulyono ;MaggyThenawidjajaSuhartono; Stella Angelina, JOHR, 2015, 3(2),125-132.
- Ruhul Amin, M.D.; Mohammed AsaduzzamanChowdhury; ArefinKowser, M.D., Heliyon , 2019, (5),1-12.
- Harunyah; Sariadi; Raudah; J. Phys. Conf. Ser., 2018,(953).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









