Experimental and Theoretical Investigations on the Corrosion Inhibition Action of Thiadiazole Derivatives on Carbon Steel in 1M Hcl Medium
Reeja Johnson1, Joby Thomas Kakkassery1* , Vinod Raphael Palayoor2, Ragi Kooliyat1 and Vidhya Thomas Kannanaikkal1
, Vinod Raphael Palayoor2, Ragi Kooliyat1 and Vidhya Thomas Kannanaikkal1
1Centre for Electrochemical Studies, St. Thomas’ College (Autonomous), Thrissur,(University of Calicut), Kerala-680001, Iindia.
2Department of Chemistry, Government Engineering College, Thrissur, Kerala-680009, India.
Corresponding Author E-mail: drjobythomask@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360624
Article Received on : 17-10-2020
Article Accepted on :
Article Published : 30 Dec 2020
Novel thiadiazole derivatives of Schiff bases namely (E)-N-(anthracen-9-ylmethylene)-5-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine (A9CNPTDA) and N-(anthracen-9(10H)-ylidene)-5-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine (ANNPTDA) were synthesized, characterized and corrosion inhibition behaviour, as well as the mechanism of inhibition were investigated by different monitoring techniques like gravimetric measurements, electrochemical impedance spectroscopy, potentiodynamic polarization, quantum chemical and SEM studies. Both of the thiadiazole derivatives showed excellent corrosion inhibitor action on carbon Steel in acid medium. A9CNPTDA exhibited highest inhibition efficiency of 98.04% at 1mM concentration while ANNPTDA showed a maximum of 95.32%. In HCl medium, both derivatives obeyed Langmuir adsorption isotherm and thermodynamic parameters (Kads, ∆G0ads) were calculated. An acceptable relationship was observed between the results of quantum chemical calculations and other corrosion monitoring analysis.
KEYWORDS:Adsorption Isotherm; Corrosion Inhibition; Electrochemical Impedance; Potentiodynamic Polarization; Schiff Bases; Thiadiazoles
Download this article as:| Copy the following to cite this article: Johnson R, Kakkassery J. T, Palayoor V. R, Kooliyat R, Kannanaikkal V. T. Experimental and Theoretical Investigations on the Corrosion Inhibition action of Thiadiazole Derivatives on Carbon Steel in 1M HCl medium. Orient J Chem 2020;36(6). |
| Copy the following to cite this URL: Johnson R, Kakkassery J. T, Palayoor V. R, Kooliyat R, Kannanaikkal V. T. Experimental and Theoretical Investigations on the Corrosion Inhibition action of Thiadiazole Derivatives on Carbon Steel in 1M HCl medium. Orient J Chem 2020;36(6). Available from: https://bit.ly/3nXUvBm |
Introduction
The acidic solutions are usually used in metal industries for de-scaling, acid pickling, cleaning process and presence of these corrosive media are the foremost reasons for the increase in the rate of metallic dissolution. The exploitation of assured organic compounds as inhibitors is the most practical method to prevent corrosion of the Carbon Steel (CS) in acidic media1,2. Compounds with π-bonds generally exhibit marked inhibitive properties due to the interaction of π-orbital with the metal surface3-6. Most of the well-known acid inhibitors in acid are organic compounds holding sulphur, oxygen and nitrogen atoms. The effectiveness of these molecules mainly depends on their ability to get adsorbed on the surface of metal particularly through polar groups. Significant amount of effort is devoted to develop efficient and novel corrosion inhibitors, in recent years7-10. Various compounds containing nitrogen, sulphur, and/or oxygen, especially certain thiadiazole derivatives have attracted more and more attention in this area. The present investigation was undertaken to examine the corrosion inhibition efficiencies of two novel thiadiazole derivatives, A9CNPTDA and ANNPTDA derived from anthracene-9-carbaldehyde and anthrone, on carbon steel using gravimetric weight loss measurements, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), quantum chemical, and SEM studies. The corrosion inhibition mechanism was confirmed by adsorption and surface morphology analysis. Quantum chemical investigations on corrosion inhibition using Gammess of to were also performed.
Materials and Methods
Inhibitor and Solutions
All chemicals (Sigma-Aldrich) and absolute ethanol (C2H5OH, Fisher, 99.9%) were used as received .The azomethine compound was synthesized and characterized through their spectral data. The aggressive solutions of 1M HCl was prepared by using standard grade HCl (Merck) and deionized water. Inhibitor solutions were prepared in the range, 0.2mM-1Mm concentrations.
Synthesis and Characterization
Polynuclear Schiff base, (E)-N-(anthracen-9-ylmethylene)-5-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine (A9CNPTDA) was synthesized by adding a hot ethanolic solution of anthracene-9-carbaldehyde (2mM) in dropwise to stirred solution containing equimolar concentration of 5-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine in ethanol medium. The mixture was refluxed for 4hr, cooled and concentrated to derive orange coloured needle-shaped crystals of A9CNPTDA.m.p 2790C (Yield: 73%)
Heterocyclic Schiff base N-(anthracen-9(10H)-ylidene)-5-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine (ANNPTDA) was synthesized by the condensation of equimolar mixture of 5-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine and anthrone in ethanol medium. The reaction mixture was refluxed for 4hr, concentrated, and cooled. Offwhitecoloured crystals of ANNPTDA separated was filtered, washed and dried and m.p2100C (Yield: 75%)
Schematic representation of the synthesis of Schiff bases (A9CNPTDA and ANNPTDA) are shown in Figure 1.Synthesized products were characterized by elemental analysis as well as electronic, FTIR, Mass and NMR Spectral studies with aid of Shimadzu UV-Visible-1800(DMSO), Shimadzu IR-Affinity-1, Shimadzu, QP 2010 GCMS and BrukerAvance III HD respectively.
 |
Figure 1: Schematic representation of synthesis of Schiff bases A9CNPTDA and ANNPTDA |
Gravimetric Studies
Carbon steel coupons were cut and abraded with various grades of SiC papers (100, 220, 400, 600, 800, 1000, 1500 and 2000) washed with distilled water followed by acetone. Approximate composition of the steel specimen was determined by EDAX technique (0.55% C,0.08% Mn,0.04% P,0.012% S,0.02% Si, and Fe the rest using Hitachi SU6600 model SEM. Stock solutions of A9CNPTDA and ANNPTDA (1mM) in 1M HCl were prepared and diluted to get different concentrations (0.2-1mM).
The metal specimens were immersed in the aggressive medium for 5 days at 28±0.10C with a periodical evaluation of the corrosion rate. The experiments were carried out in duplicate and the average values were reported. The rate of corrosion was determined by11,12

where𝜐= corrosion rate (mmy−1), 𝑊= weight loss (g), 𝑆= surface area of metal specimen (cm2), 𝑡= time of treatment (h), 𝐷= density of specimen (gcm−3), and 𝐾= a constant (8.76 × 104). The inhibition efficiency (%) was obtained by the following equation:

where𝜐&𝜐’are the corrosion rate of the metal specimen in the absence and presence of the inhibitor, respectively.
Effect of Temperature
The effect of temperature on corrosion was evaluated by the gravimetric weight loss studies at the temperature range 30-60°C. Arrheniusequation13 was used for the calculation of the activation energy of corrosion with and without inhibitors which is given by,

whereK is the rate of corrosion, A the frequency factor, Ea the activation energy andT the temperature in Kelvin and R is the gas constant. Arrhenius curves were obtained by plotting logK against 1000/T. Enthalpy and entropy of activation(∆H*, ∆S*) were calculated from the transition state theory, which can be represented by the following equation.

Electrochemical Studies
Electrochemical studies were executed using three-electrode cell assembly consisting of CS as working electrode (exposed area 1cm2), platinum electrode as counter electrode (1cm2) and saturated calomel electrode (SCE) as reference electrode at 280C. For acid corrosion, the working area of metal specimens was made to contact with the electrolyte for 30min preceding the experiment. IviumCompactstat-e electrochemical system together with Iviumsoft software package was used to perform the experiments.
Electrochemical Impedance Spectroscopic Studies (EIS)
EIS measurements were taken at the constant potential in the frequency range from 1 KHz to 100mHz with an amplitude of 10mV as excitation signal. The percentage of inhibition was calculated by the equation14,

whereRct and R’ct are the charge transfer resistances of the working electrode in the presence and absence of inhibitor, respectively.
Potentiodynamic Polarization Studies
Polarization studies were carried out by altering the potential of working electrode from +250 to -250 mV with a sweep rate of 1mV/s15. Slope analysis of Tafel curves gave the corrosion current densities and the inhibition efficiency (ƞpol%) was calculated

whereIcorr andI’corr are corrosion current densities of the working electrode in the absence and presence of inhibitors,respectively.
Surface Morphological Studies
Scanning Electron Microscope images were recorded using Hitachi SU6600 model scanning electron microscope, after treatment with 1M HCl solution with and without the inhibitors, for 24hr.
Quantum Chemical Studies
GAMMES software and DFT method were employed for the determination of optimized geometry of compounds and quantum chemical evaluations. A combination of Lee–Yang–Parr nonlocal correlation functional (B3LYP), and Beck’s three-parameter exchange functional were employed in DFT method16-18.
Results and Discussion
The structures of inhibitor molecules synthesized were confirmed by spectroscopic and elemental data.
and elemental data.
N-(anthracen-9-ylmethylene)-5-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine (A9CNPTDA)
Anal.calcd. for C23H14N4O2S: C,67.30; H, 3.44; N,13.65; O,7.8; S,7.81%. Found: C,66.72; H,3.31; N, 13.58; O, 7.5; S, 7.75%. IR(KBr); 1691cm-1(C=N),3102cm-1(C-H),1556 and 1445cm-1(C=C),1527cm-1,1347cm-1(NO2),1274cm-1(C-N),1104cm-1(C-S), UV; 39062cm-1(π→π*),27100cm-1(n→π*), 1HNMR; δCH=N 11.5(s),δNH12.0(br), δCH in aromatic rings 7.75- 9.03(m).13C NMR; 136ppm (C=N), 123-154ppm (aromatic C). EIMS; m/z 222(base peak), [C8H6 N4O2S]+, 163[C8H7N2S]+, 120[C7H4S] +, m/z 91[C7H7] + and 74[CH2N2S] +.
N-(anthracen-9(10H)-ylidene)-5-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine (ANNPTDA)
Anal.calcd. forC22H14N4O2S: C,66.32; H,3.54; N,14.06; O,8.03; S,8.05%. Found: C,65.72; H,3.38; N,13.84; O,7.4; S,7.95%. IR(KBr); 1662cm-1(C=N),3066cm-1(C-H), 1597,1510cm-1(C=C), 1317,1400cm-1(NO2), 1166cm-1(C-N),1101cm-1(C-S), UV; 39062cm-1,37453cm-1(π→π*), 27174cm-1(n→π*), 1HNMR; δCH24.3(s), δCH in aromatic rings 7.34-8.32(m).13C NMR; 123-164 ppm (18 different aromatic sp2 C atoms).EIMS; m/z 194(base peak), [C14H12 N]+,165[C8H9N2S]+.
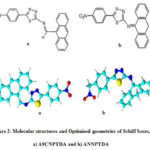 |
Figure 2: Molecular structures and Optimised geometries of Schiff bases, a) A9CNPTDA and b) ANNPTDA |
Gravimetric Corrosion Inhibition Studies
The corrosion inhibition efficiencies of two molecules A9CNPTDAand ANNPTDA for 24hr at (28±0.10C) are listed in Table 1. Both molecules displayed excellent inhibitionefficiency on CS surface, maybe due to the presence of highly polarizablesulphur atom and nitrogen atom in the thiadiazole ring.Presence of azomethine linkage and aromatic rings increases its inhibition efficiency even at very low concentration. For a period of 24hr, A9CNPTDA showed higher inhibition efficiency than ANNPTDA.The remarkable corrosion preventing ability of A9CNPTDAmay be attributed to the presence of highly conjugated azomethine linkage and planarity of the molecule. In addition to this highly delocalized π electron cloud of the aromatic ring increases its inhibition efficiency. Even though there is a structural similarity between A9CNPTDA and ANNPTDA, corrosion inhibition efficiency of ANNPTDA is relatively low, compared to that of A9CNPTDA which may be due to thedeviation from coplanarity of the molecule. Presence of strained azomethine linkage and less aromatic nature in the molecule also decrease its inhibition efficiency.
Table 1: Gravimetric corrosion inhibition efficiencies (%) of A9CNPTDA and ANNPTDA on CS in 1M HCl for 24hr at 28±0.10C
|
Conc. (mM) |
A9CNPTDA |
ANNPTDA |
|
0.2 |
93.87 |
81.68 |
|
0.4 |
94.26 |
87.16 |
|
0.6 |
95.42 |
89.98 |
|
0.8 |
96.17 |
91.51 |
|
1.0 |
98.04 |
95.32 |
Figure 3 represents the variation of corrosion inhibition efficiency with time for the inhibitor molecules at 1mM concentration. The plot reveals that the decrease of 𝜂𝑤% for A9CNPTDA was very much steeper than that of ANNPTDAas the days went on. This may be due to excellent adsorption of these molecules on the metal surface because of the presence of highly active heteroatoms and slow hydrolysis rate of A9CNPTDA molecules in acidic medium. The azomethine linkage (C=N) present in the molecule is susceptible to hydrolysis.The stability of the inhibitor in the aggressive medium is very important if it is to be recommended for long time use. From the results of gravimetric corrosion studies, one can reach into the conclusion that A9CNPTDA molecule acts as an excellent corrosion inhibitor for a long time.
 |
Figure 3: Variation of corrosion inhibition efficiencies of A9CNPTDA andANNPTDA (1mM) with time on CS in 1MHCl |
Effect of Temperature
It is apparent from the results that activation energy of dissolution of metal increased with inhibitor concentration. Table 2 explores the activation energy and thermodynamic parameters of corrosion of CS in 1M HCl in the presence and absence of A9CNPTDA and ANNPTDA respectively.
Table 2: Thermodynamic parameters of CS corrosion, in the presence and absence of Schiff base inhibitors, A9CNPTDA and ANNPTDA in 1M HCl
|
Inhibitors |
C |
Ea |
A |
∆H* |
∆S* |
|
(mM) |
(kJ mol-1) |
(kJ mol-1) |
(J mol-1 K-1) |
||
|
Blank |
51.33 |
8.73 x109 |
48.7 |
-56.51 |
|
|
|
0.2 |
109.23 |
1.48 x1019 |
107 |
107.29 |
|
0.4 |
117 |
2.34 x1020 |
114 |
110.16 |
|
|
A9CNPTDA |
0.6 |
128.7 |
1.91 x1022 |
126 |
113.04 |
|
0.8 |
132 |
8.71 x1022 |
130 |
121.26 |
|
|
1.0 |
140 |
1.2 x1024 |
138 |
133.33 |
|
|
0.2 |
94.45 |
6.46×1016 |
91.8 |
75.12 |
|
|
0.4 |
97.57 |
2.0×1017 |
94.9 |
84.51 |
|
|
ANNPTDA |
0.6 |
102.82 |
1.38×1018 |
100 |
100.39 |
|
|
0.8 |
109.1 |
1.39×1019 |
106 |
119.55 |
|
|
1.0 |
116.83 |
2.69×1020 |
117 |
142.71 |
Positive signs of enthalpies with a regular rise reflect the endothermic nature of dissolution and the increasing difficulty of corrosion with the inhibitor. Increase of entropy values emphasizes that an increase for the activated complex take place than the reactant with the concentration of the inhibitor. This implies that the reluctanceof dissolution of metal increased with the inhibitor concentration, whichcan be attributed to the considerable intervention of inhibitor molecules during the metallic dissolution.
Electrochemical Impedance Spectroscopic Studies (EIS)
The corrosion behaviour of CS in 1M HCl with and without inhibitor was examined using impedance spectroscopic analysis at 280C. Figures4 and 5 represent the Nyquist plots and Bode plots of two Schiff bases A9CNPTDA and ANNPTDA respectively. Impedance parameters including double-layer capacitance (Cdl), solution resistance (Rs) and percentage of inhibition efficiency(ηEIS%) were evaluated from the values of charge transfer resistance(Rct) and tabulated in Table 3. At both higher and lower frequencies, the capacitance loop intercepts the real axis. These intercepts at the high-frequency end represent the solution resistance (Rs) and at the lower frequency end denote the sum of Rs and Rct. The difference between these intercepts can be calculated to find out Rctwhich is a measure of the electron transfer takes place on the exposed metallic surface analysis and is inversely proportional to the corrosion rate of the surface19,20.The equivalent circuit that exactly fit the EIS curves generally consisted of a solution resistance Rs, double-layer capacitance and charge transfer resistance Rct(Figure 6). Table 1, reveals that as increasing concentration of both inhibitors, Rct values were increased and Cdl values were decreased.
The variation in Rct values can be explained by the adsorption process by which the inhibition mechanism takes place21. As the inhibitor concentration increases there would be a considerable increase in the amount of adsorption process which results in the prevention of the charge transfer of the metal atoms on the metallic surface and solution, which in turn increase the charge transfer resistance. The lowering of Cdl values with increase in the inhibitor concentration can be associated with the reduction of local dielectric constant values and rise in the thickness of the electrical double layer. These observations testify the inhibitor action at the solution–metal interface. From the data, it is obvious that both Schiff bases A9CNPTDA and ANNPTDA act as potential corrosion inhibitors in hydrochloric acid medium. Maximum efficiency of inhibition 97.90% was obtained for A9CNPTDAand 92.47% for ANNPTDA at 1mM concentration
Table 3: Electrochemical impedance parameters of CS corrosion with and without Schiff bases A9CNPTDA and ANNPTDA in 1M HCl
|
Inhibitors |
Conc. (mM) |
Cdl (µF cm2) |
Rct (Ω cm-2) |
ηEIS% |
|
Blank |
101 |
16.1 |
– |
|
|
0.2 |
83.9 |
157 |
89.74 |
|
|
A9CNPTDA |
0.4 |
53.0 |
187 |
91.39 |
|
0.6 |
45.7 |
239 |
93.23 |
|
|
0.8 |
47.5 |
712 |
97.73 |
|
|
1.0 |
53.1 |
768 |
97.90 |
|
|
0.2 |
84.3 |
29 |
61.80 |
|
|
ANNPTDA |
0.4 |
46.0 |
38.8 |
83.50 |
|
0.6 |
73.1 |
68.6 |
85.09 |
|
|
0.8 |
74.9 |
131 |
90.69 |
|
|
1.0 |
73.6 |
208 |
92.47 |
 |
Figure 4: a) Nyquist plots and b) Bode plots of CS in the presence and absence of A9CNPTDA in1M HCl |
 |
Figure 5: a) Nyquist plots and b) Bode plots of CS in the presence and absence of ANNPTDA in1M HCl. |
 |
Figure 6: Randles circuit fitted for EIS measurements. |
Potentiodynamic Polarization Studies
Tafel extrapolation analysis and linear polarization studies were conducted to establish the impact of Schiff bases towards the polarization of metal specimens by the determination of polarization resistance, corrosion current density and the percentage of inhibition efficiencies. Tafel and linear polarization curves recorded are represented in Figures7 and 8. The corrosion parameterslike corrosion potential(Ecorr), corrosion current density(Icorr), polarization resistance(Rp) and inhibition efficiency(ηpol%) are listed in Table 4.
Table 4: Potentiodynamic polarization parameters of CS in the presence and absence of A9CNPTDAand ANNPTDA in 1M HCl at 280C for immersion perio, 30 min
|
Inhibitors |
Conc. (mM) |
𝐸corr (mV/SCE) |
𝐼corr (𝜇A/cm2 ) |
– bc |
𝑏𝑎 |
𝜂pol% |
|
A9CNPTDA |
Blank |
451 |
1115 |
110 |
184 |
– |
|
0.2 |
471 |
77.0 |
70 |
134 |
93.1 |
|
|
0.4 |
469 |
37.8 |
63 |
119 |
96.6 |
|
|
0.6 |
479 |
36.0 |
61 |
123 |
96.8 |
|
|
0.8 |
470 |
30.3 |
66 |
115 |
97.3 |
|
|
1.0 |
476 |
12.2 |
52 |
103 |
98.9 |
|
|
ANNPTDA |
0.2 |
473 |
492 |
93 |
151 |
55.9 |
|
0.4 |
468 |
161 |
83 |
153 |
85.7 |
|
|
0.6 |
472 |
125 |
73 |
142 |
88.8 |
|
|
0.8 |
453 |
105 |
62 |
138 |
90.6 |
|
|
1.0 |
483 |
70.4 |
79 |
125 |
93.7 |
From Tafel data, it is evident even at low concentrations; both the inhibitors possess marked inhibition efficiency in 1M HCl. The maximum inhibition efficiency of 98.90% and 93.70% were respectively shown by A9CNPTDA and ANNPTDA at 1mM concentration. This is also congruent with the data obtained in gravimetric analysis and EIS measurements. In both cases, the cathodic slopes exhibited predominant changes than the anodic slopes which are a clear evidence for the preferential adsorption of these Schiff bases on the cathodic sites. Furthermore, the Ecorr values were not altered significantly with respect to that of blank and appreciable change was noted in anodic or cathodic slopes in both the Schiff bases suggesting that in 1M HCl both Schiff bases behave as mixed-typeinhibitors. In other words, the hydrogen evolution process was considerably hindered by both molecule compared to the oxidationof Fe into Fe2+
 |
Figure 7: a)Tafel plots and b) Linear polarization plots for CS in the presence and absenceof A9CNPTDA in 1M HCl. |
 |
Figure 8: a) Tafel plots and b)Linear polarization plots for CS in the presence and absence of ANNPTDA in 1M HCl. |
Adsorption Studies
The mechanism of corrosion inhibition of the Schiff bases understudy was established by the adsorption studies by invoking adsorption isotherms such as Langmuir, Temkin, Frumkin and Freundlich isotherms22-26. Evaluation of adsorption parameters is done by selecting the best-fit isotherm model assisted by the correlation coefficient (R2). Both Schiff bases, A9CNPTDA and ANNPTDA obeyed Langmuir adsorption isotherms in1M HCl, which can be expressed as

where C is the concentration of inhibitor, θ is the fractional surface coverage and Kadsis the value of adsorption equilibrium constant.The adsorption equilibrium constant, Kads mainly depends on the standard free energy of adsorption ∆G0ads, by the relation

where 55.5 is the molar concentration of water, R is the ideal gas constant and T is the temperature in Kelvin. Adsorption parameter values derived are listed in Table 5.
Table 5: Adsorption parameters of Schiff bases, A9CNPTDA and ANNPTDA on CS surface in 1M HCl
|
Thermodynamic parameter |
A9CNPTA |
ANNPTDA |
|
Kads |
58823.5 |
22222.2 |
|
∆G0ads(KJ/mol) |
-37.56 |
-35.12 |
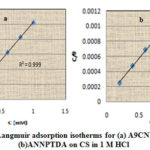 |
Figure 9: Langmuir adsorption isotherms for (a) A9CNPTDAand (b)ANNPTDA on CS in 1 M HCl |
Data reveal that A9CNPTDA has comparatively high Kads value than ANNPTDA which establishes the greater efficiency of adsorption of A9CNPTDA over ANNPTDA. The ∆G0ads values obtained for A9CNPTDA and ANNPTDA are-37.56 and -35.12KJ/mol respectively. The ∆G0ads values up to -20kJ mol-1 indicates clearly of the electrostatic attraction or physisorption between the charged molecule and charged metal surface whereas those more negative than -40kJ mol-1 indicates the strong adsorption of the inhibitors on the metallic surface through strong co-ordinate bonds or chemisorptions. In the present study, both inhibitors exhibit ∆G0 values between -20kJ mol-1and -40kJ mol-1 which indicates that the absorption behaviour of the inhibitors involves both chemical and electrostatic interaction.
Surface Morphological Studies
Surface morphology of the metal surfaces was evaluated with the aid of SEM analysis27,28. Figures 10(a)–10(d), respectively, show the magnified surface images of bare metal, metal immersed in acid, and metal treated with acid in the presence of A9CNPTDA and ANNPTDA (1mM) for 24hr. The pits and cracks appearing in the SEM image of the bare metal specimen were due to the polishing effects. The SEM image of a blank specimen appeared to be very rough due to severe corrosion. Comparatively smoother images were displayed by metal specimens immersed in 1M HCl in the presence of A9CNPTDA and ANNPTDA at 1mM concentration and the textures of all surface images showed considerable differences. This can be attributed to the prevention of CS corrosion in acid by the adsorption of molecules
 |
Figure 10: SEM images of (a) bare CS (b) CS treated with 1M HCl (c) CS treated with A9CNPTDA(1mM)(d) CS treated with ANNPTDA(1mM) for 24hr. |
Quantum Chemical Studies
The corrosion inhibition response of organic molecules can be correlated with the energy of frontier molecular orbitals. The donor-acceptor interactions (HSAB concept) between the filled molecular orbitals of the inhibitor molecules and the vacant d-orbitals of Fe atoms on the surface are very important in the prevention of metal dissolution. The highest value of EHOMO and the lowest value of ELUMO and the energy difference between the HOMO and the LUMO (∆E) are the important quantum chemical parameters which facilitate the strong binding of the molecule on the metal surface. Optimisations of geometry and quantum chemical calculations were performed by DFT method using GAMMES software. B3LYP method was employed in DFT calculations29,30. Figure 11 shows HOMO and LUMO for the studied compounds. SinceHOMO is spread throughout the compound, it is necessary to examine the coefficients of the HOMO for better understanding. The coefficient values give the information about theatomic orbital with the highest contribution and therefore the atom that has the highest tendency to donate electrons. The LUMO is the unoccupied orbital that has the lowest energy and gives information andgives information about the regions in a molecule which has the highest tendency to accept electrons from an electron-rich species.
 |
Figure 11: HOMO and LUMO of A9CNPTDAand ANNPTDA |
The energy of the HOMO (EHOMO) provides information about the tendency of a molecule to donate electrons to an electron-poor species. The higher the EHOMO, the greater is the tendency of a molecule to donate its electrons to the electron-poor species. Therefore a comparisonof the EHOMO of the studied compounds obeys the order, A9CNPTDA>ANNPTDA, which is in full accordance with the order of inhibitionefficiency which implies that A9CNPTDA has highest tendency to donate its electrons to the metal surface and therefore binds strongly on the metal surface
The energy gap between the frontier orbitals ΔEis usually very important todescribe the static molecular reactivity. In this way, low values of ΔE imply that the polarization and theadsorption of the molecule on the metal surface is much easy. However, as it is shown in Table 6, A9CNPTDA exhibits a smaller ΔE (2.68eV) than ANNPTDA (3.02eV), indicating the high ability to accept electrons from the d-orbital of iron and high stability of the [Fe–L] complexes
Table 6: Quantum chemical parameters of A9CNPTDA and ANNPTDA
|
Schiff base |
EHOMO (eV) |
ELUMO (eV) |
∆E |
𝜒 |
𝜂 |
∆ N |
|
A9CNPTDA |
-3.530 |
-0.8435 |
2.68 |
2.204 |
1.361 |
0.836 |
|
ANNPTDA |
-3.565 |
-0.5442 |
3.02 |
2.041 |
1.497 |
0.815 |
The number of electrons transferred (∆N) indicates the tendency of a molecule to donate electrons. The higher the value of ∆N, the greater is the tendency of a molecule to donate electrons to the electron-poor species. In the case of corrosion inhibitors, a higher ∆N implies a greater tendency to interact with the metal surface through the adsorption process and A9CNPTDA has relatively ∆N value. According to Lukovits31,32, if ΔN < 3.6, the inhibition efficiency is increased with increasing electron-donating ability at the steel surface. In this study, synthesised inhibitors were the donor of electrons, and the metal surface was the acceptor. This result supports the assertion that the adsorption of inhibitors on the steel surface can occur based on donor-acceptor interactions between the π electrons of the compound and the vacant d-orbitals of the metal surface. The essential effect of inhibitors is due to the presence of free electron pairs in the N and S atoms, p-electrons on the aromatic ring, type of interaction with the steel surface, and metallic complexes formation. It is well known that steel has potential coordination affinity towards N and S donors Schiff bases. Therefore, adsorption on the metal surface can be ascribed to coordination through heteroatoms and π-electrons of aromatic rings33. In the present case, synthesized inhibitors have unshared electron pairs on N and S which enable them to form coordination with steel.
Conclusion
Two novel polynuclear Schiff bases A9CNPTDA and ANNPTDA are very effective corrosion inhibitors for CS in HCl medium. The inhibition efficiency of both Schiff bases was exceeded 95%, which may be due to the presence of highly polarisable ‘S’atom, lone pair of electrons on N atom, aromatic rings and azomethine linkage etc; All corrosion monitoring studies revealed that the inhibition efficiency of ANNPTDA is lower than that of A9CNPTDA at all concentrations. It may be due to the presence of strained azomethine linkage, relativelyless aromatic behaviour and slight deviation from thecoplanarity may be attributed to the decreased inhibition efficiency of ANNPTDA.Both inhibitors obeyed Langmuir adsorption isotherm on the CS surface during the inhibition process. The inhibitors delay the process of corrosion by hindering the cathodic process mainly. Quantum chemical studies differentiated the protective power of molecules against CS corrosion. Surface analysis using SEM portrayed the anticorrosive capacity of inhibitors.
Acknowledgement
The authors are grateful to SAIF-STIC, Cochin University of science and technology for their valuable support.
Conflicts of Interest
The author declares no conflict of interest.
References
- Agrawal, R.;Namboodhri T.K.G. Corros. Sci. 1990,30, 37-52.
CrossRef - Sekine, I.A.;Masuko, A.; Senoo, K. Corros. Sci.1987, 43, 553-562.
CrossRef - Quraishi, M.A.; Khan, M.A.W.; Ajmal, M. Anti-Corros. Methods Mater. 1996, 43, 5-21.
CrossRef - Murlidharan, S.;Iyer S.V.K. Anti-Corros. Methods Mater. 1997, 44, 100-118.
CrossRef - Al-Andis, N.; Khamis, E.; Al-Mayouf, H.; Aboul, B. Corros. Prev. Control. 1995, 42, 13-25.
- Hammouti, B.; Aouniti, M.;Taleb; Brighli, M.; Kertit, S.; Corrosion. 1995, 51,441-461.
CrossRef - Begum, A.S.; Nalini, D.; and Devi, T.M. Orient. J. Chem. 2010,26(4), 1333-1343.
- Bhagat,S.M; Bhagat,T.M; Unchandkar,A.R; Deshpande, M.N.; Orient. J. Chem. 2010
26(4), 1545-1548. - Bhagat, S.M.; Kolhatkar, D.G.; Anchdkar,A.R.; and Deshpande, M. N. Orient. J. Chem. 2010, 26(4), 649-653.
- Begum, A.S.; Nalini,D.; Suvarnna,K. Orient. J. Chem. 2010, 26(3), 891-900.
- Deng, S; Li, X; Fu, H. Corros. Sci. 2011, 53, 3704-3711.
CrossRef - Emregul, K.C; Atakol,O. Mater. Chem. Phys. 2004, 83, 373-379
CrossRef - Musa, A.Y ; Kadhum, A.A.H.; Mohamad, A. B.; Takriff, M. S. Corros. Sci. 2010, 52(10), 3331–3340
CrossRef - Ashassi-Sorkhabi, H; Shaabani, B; Seifzadeh, D. Electrochem. Acta. 2005, 50, 3446-3452.
CrossRef - Bentiss,F.; Lagrenee, M.; Traisnel, M.; Hornez, J. C. Corrosion. 1999,55(10), 968–976.
CrossRef - Reza Soleymani, Khalil Ghesmat-Konandeh, ReihanehDehghanianDijvejin, Orient. J. Chem.2012,28 (3), 1331-1346.
CrossRef - Reza Soleymani, Sahar Farsi-Madan, Khalil Ghesmat-Konandeh. Orient. J. Chem.2012, 28 (2), 703-715.
CrossRef - Reza Soleymani, Yasin Mohammad Salehi, TaherehYousofzad, Maryam Karimi-Cheshmeh Ali. Orient. J. Chem. 2012, 28 (2), 627-643.
- Yurt, A.; Balaban, A.; Kandemir, S.U.; Bereket, G.; Erk, B. Mater. Chem. Phys. 2004, 85,420-426.
CrossRef - McCafferty, M.; Hackerman, N.; J. Electrochem. Soc.1972, 119,146-154.
CrossRef - Azhar, M.E.; Mernari, B.; Traisnel, M.; Bentiss, F.; Lagrenee, M. Corros. Sci. 2001, 43, 2229-2238.
CrossRef - Cottis, R.A; Al-Awadhi, H.; Al-Mazeedi; Turgoose, S. Electrochem.Acta. 2001,46( 24), 3665–3674.
CrossRef - Hamani, H.; Douadi, T.; Daoud, D.; Al-Noaimi, M.; Chafaa, S.; Measurement. 2016, 94,837-846
CrossRef - Ateya, B.G; El-Anadouli, B.E; El-Nizamy, F.M,Corros. Sci. 1984, 24(6),509-515.
CrossRef - Zhao, T.; Mu, G, Corros. Sci. 1999; 41(10), 1937-1944.
CrossRef - Soltani, N, Salavati, H, Rasouli, N, Paziresh, M, Moghadasi, A. Chem. Eng.Com. 2016, 203(6), 840-854.
- Palayoor V.R.; Kakkassery, J.T.; Kanimangalath S.S.; Varghese S. Int. J. Ind.Chem. 2017,8(1), 49-60.
CrossRef - Ashassi-Sorkhabi, H, Shaabani, B, Seifzadeh D. Appl.Surf.Sci. 2005,239(2),154-164.
CrossRef - Sastri,V.S.; Perumareddi, J.R.; Corrosion. 1997, 3(8), 617-22.
CrossRef - Senet.P.; Chem.Phys. Lett.1997, 275( 5), 527–532.
CrossRef - Lukovits, I.; Kalman, E.; Zucchi,F, Corrosion. 2001,7(1), 3–8.
CrossRef - Li, L.; Zhang, X.; Lei, J.; He, J.; Zhang, S.; Pan, F.; Corros. Sci.2012,63, 82–90.
CrossRef - Zheng, X.; Zhang, S.; Li, W.; Gong, M.; Yin, L.;Corros. Sci. 2015,95, 168–179.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









