Nano drug delivery Study of Anticancer Properties on Jackfruit using QM/MM Methods
Behnaz Bonsakhteh*, Abdolhossein Rustaiyan
Department of Chemistry, Tehran Science and Research Branch, Islamic Azad University, Tehran, Iran
DOI : http://dx.doi.org/10.13005/ojc/300430
Article Received on :
Article Accepted on :
Article Published : 18 Dec 2014
Nano-biotechnology takes most of its fundamentals from nanotechnology which most of the equipment designed for nano-biotechnological are based on other existing nanotechnologies. Nano-biotechnology is often used to describe the overlapping multidisciplinary activities associated with chemistry, biology and nano-medicine. In this investigation, the ab initio calculations were implemented using Gaussian program package based on density functional level of theory (DFT) to achieve the drug delivery technic for unraveling of linkage of Jackfruit to single walled carbon nanotubes . NMR investigation gives deeper physical insight into the impact of different structures . In this work NMR parameters were calculated at the Ethyl isovalerate, Propylisovalerate, Isobutyl isovalerate and 3-methyl butyl acetate extracted of Jack fruit with different functional groups in their active sites so, the anticancer properties of this compound have been clarified.
KEYWORDS:Jackfruit; CNT; anticancer; NMR; DFT; effective compounds
Download this article as:| Copy the following to cite this article: Bonsakhteh B, Rustaiyan A. Nano drug delivery Study of Anticancer Properties on Jack fruit using QM/MM Methods. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Bonsakhteh B, Rustaiyan A. Nano drug delivery Study of Anticancer Properties on Jack fruit using QM/MM Methods. Available from: http://www.orientjchem.org/?p=6024 |
Introduction
The Jackfruit belongs to the Mulberry family that is native to parts of Southern and Southeast Asia. It can also be found in East African and Brazil. The fruit is the largest tree-borne fruit in the world. The Jackfruit is has an abundance of vitamin C which helps to protect against viral and bacterial infection [1-5]. It helps in strengthening the immune system as it support white blood cell function. Vitamin C is widely known for its ability to prevent colds and flu. Being rich in phytonutrients like Lignans, Isoflavines and Saponins that are all known for their anti-cancer and anti-aging properties, the Jackfruit helps in destroying cancer-causing free radicals and slows down the degeneration of cells that leads to degenerative diseases. These phytonutrients protect the skin and help to keep it supple and smooth. The potassium in the Jackfruit is found to help in lowering blood pressure and reverses the effects of sodium that causes a rise in blood pressure that affects the heart and blood vessels [6-10]. This helps in preventing heart disease and strokes. Potassium also helps in preventing bone loss and improves muscle and nerve function. Another heart friendly property found in the Jackfruit is due to vitamin B6 that helps reduce homocysteine levels in the blood thus lowering the risk of heart disease. The high fiber content of Jackfruit prevents constipation as it bulks up food consumed which then passes more easily through the gut [11-16].The fiber also offers protection against colon mucus membrane by mopping up carcinogenic chemicals in the large intestine and preventing colon cancer. The anti-ulcer properties help cure ulcers and other digestive disorders [17-20]. Certain extracts from the root of the Jackfruit plant are said to help cure diarrhea and found to be beneficial for those suffering from asthma. With a small amount of Vitamin A available, it helps maintain good vision and smooth skin. Vitamin A is instrumental in preventing age related macular degeneration and night blindness. The Jackfruit contains iron that helps prevent anemia and aids in proper circulation, magnesium that is important in the absorption of calcium. Calcium is important in the development strong teeth and bones and helps prevent bone related disorders like osteoporosis. It has copper that helps maintain a healthy thyroid and plays an important part in hormone production and absorption [21-24]. The calcium contained in the fruit also helps in the clotting of blood. With a large amount of simple sugars like fructose and sucrose, it boosts energy and as it contains no fat or cholesterol, it is perfect as a diet food. This exotic fruit is a great fruit and can be served raw or cooked but either way it has many health benefits [25,26].
Biopolymers are polymers which are produced from renewable natural sources, are often biodegradable, and not toxic to generate [27,28]. They offer the benefits of being bio-degradable, non-toxic and ability to bind with a number of drugs. They can be manufactures into a number of pharmaceutical compounds and hence be used as novel drug delivery carriers. Jackfruit contains morin, carotenoids, provitaminA[29-32]. It is used medicinally asa laxative, tonic and demulcent. In more recent years many drugs have been shown to achieve a better systemic bioavailability by self medication through the oromucosal route than by oral administration[33-38].
It is now widely accepted that the beneficial effects of fruits and vegetables for the prevention of certain diseases are due to the bioactive compounds they contain [39-42].
Recent years have seen increased interest on the part of consumers, researchers, and the food industries into how food products can help maintain health; and the role that diet plays in the prevention and treatment of many illnesses has become widely accepted . The aim of this review was to present an overview of the functional, medicinal, and physiological properties of the Jackfruit[43 -45].
Drug delivery is a rapidly growing area that is now taking advantage of nanotube technology. These nanotubes function with a larger inner volume to be used as the drug container, large aspect ratios for numerous functionalization attachments, and the ability to be readily taken up by the cell[46-50].
In this work, we have used computational chemistry techniques to investigate current problems in biology, medicinal chemistry and materials research. The group also develops novel methods for predicting biological activity and molecular properties based on quantum mechanics (QM) methods. Due to this purpose ,we have studied the chemical properties of effective structures in Jackfruit consisting of Ethyl isovalerate ,Propyl isovalerate ,Isobutyl isovalerate and 3-methyl butyl acetate using theoretical and computational methods [51-54].
Computational Methods
In this study, we have investigated various properties of effective compounds of Jackfruit extract. This molecule has effective role in anticancer characteristic of Jackfruit. Ab-initio calculations were carried out by density functional theory method (DFT). This showed the significant role of different atoms of mentioned compound on anti-cancer characteristics of Jackfruit.
The chemical shift refers to phenomenon which associated with the secondary magnetic field created by the induced motions of the electrons that surrounding the nuclei when in the presence of an applied magnetic field. . [55-57]. The shielding σ, is the differential resonance shift due to the induced motion of the electrons. The chemical shielding tensor is commonly referred to the chemical shift anisotropy (CSA) tensor according to the possession of second rank properties. The CSA tensor can be described by three additional parameters [58].
a) The isotropic value(σiso) , of the shielding tensor which can be defined as: [59-61].
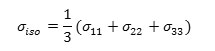
b) The chemical shift anisotropy parameter (∆σ), due to the following expression:
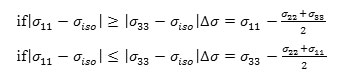
and
c) The asymmetry parameter (ƞ), which has given by
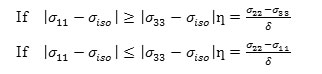
It is useful to define the span (Ω), and the skew (ĸ) of a CSA tensor. The span is defined as:
![]()
and indicates the width of the NMR line shape for a nonspinning, stationary, sample . The skew is defined as:
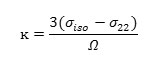
The main structures in Jackfruit Ethyl isovalerate, Propyl isovalerate, Isobutyl isovalerate and 3-methyl butyl acetate with effective sides including different atoms [62-64].
Using the theoretical software, it is possible to show the chemical structure of the molecules that have main role in the anti-cancer effect of Jackfruit. NMR parameters have calculated by Gaussian 03 program at DFT level of theory [65].
 |
Table1: NMR parameters (ppm) of ethyl-n-propyl disulphide ,ethanetiol, diethyl trisulphide, diethyl disulphide,propyl-2-methyl-butanoate,ethyl-2-methyl butanoate, ethyl propanoate using DFT level of theory Click here to View table |
Results and Discussion
Nuclear Magnetic Resonance
study depicts the physical insight into the influence of different structures.In this work NMR parameters were calculated the Ethyl isovalerate, Propyl isovalerate, Isobutyl isovalerate and 3-methyl butyl acetate extracted of Jackfruit with different functional groups in their active sites that the consider models geometry optimization were accomplished by method DFT by standard basis set (6-31 G*) are employed to calculate the CS tensor. The CS tensors have calculated the sites of various atoms [66-69].
One of the powerful computational methods is Density functional theory (DFT), which it has applied to investigate on these epileptic drugs. In fact ,we calculate NMR factors and then compare data and We draw diagrams associated with them According to Fig.1[70-72].
 |
Fig1: NMR parameters of Ethylisovalerate ,Propyl isovalerate ,Isobutyl isovalerate and 3-methyl butyl acetateversus different atoms in active sites using DFT level of theory Click here to View figure |
Therefore, we resulted that Ethyl group substitution causes the stability of course, highlighting the vital role of Jackfruit and the Phenyl group substitution can scramble to reduce the role of atoms influences Jackfruit [73,74].
NMR calculations on Jackfruit using density functional theory (DFT) reveal that methods including electron correlation show significant improvements in the NMR shielding over results. The NMR measurements were carried out using B3LYP/6-31G* in GIAO method of nuclear magnetic resonance at theoretical concepts in different dielectric constants (Table1) [75,76].
NMR calculations on Jackfruit using density functional theory (DFT) reveal that methods including electron correlation show significant improvements in the NMR shielding over results (Table 1)[77-79].
The results of table1 are shown in fig.1 , where we plot the chemical shift(δ) and chemical shift isotropy (σiso) , asymmetry parameter (η) and shielding tensor anisotropy (Ω) and anisotropy (∆σ)of the Ethyl isovalerate ,Propyl isovalerate ,Isobutyl isovalerate and 3-methyl butyl acetate extracted of Jackfruit with different functional groups in their active sites [80,81].
We have found that the O and S atoms denoted has maximal shift in all compounds and other indicated atoms almost have the similar shifts in different structures (Fig.1). This shows the significant role of these atoms, in the above mentioned molecules, on anti-cancer characteristics of Jackfruit [82-84].
Also, the graphs of HNMR and CNMR based on isotropic magnetic shielding constants σiso (ppm), anisotropic magnetic shielding tensors σaniso (ppm) and Chemical shifts δ (ppm) for effective compound -CNT systems are listed in Figs2,3 [85-88].
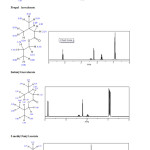 |
Fig2: Results of NMR 1H for Ethylisovalerate ,Propyl isovalerate ,Isobutyl isovalerate and 3-methyl butyl acetate. Click here to View figure |
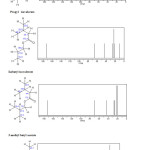 |
Fig3: Results of NMR 13C for Ethyl isovalerate ,Propyl isovalerate ,Isobutyl isovalerate and 3-methyl butyl acetate.CNT (5,5 )Armchair-Ethyl isovalerate CNT (6,6)Armchair– Propyl isovalerate Click here to View figure |
The results of the above observations strongly suggest that the different data observed in the effective compounds- SWCNT is predominantly due basis set functions, induced by a change in active sides of the various structures (Fig.4) [89-93].
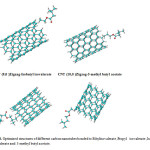 |
Fig4: Optimized structures of different carbon nanotubes bonded to Ethylisovalerate ,Propyl isovalerate ,Isobutyl isovalerate and 3-methyl butyl acetate. Click here to View figure |
Conclusion
Use of the different structures for characterization of motions and determination of the properties or dynamics of the molecules of interest requires a number of theoretical or computational steps and all of which are current activities of research, Therefore in this paper we summarize the method and describing the reasons for the choices.
To conclude, we have performed simulations and solvent NMR of theoretical methodology on Ethyl isovalerate ,Propyl isovalerate ,Isobutyl isovalerate and 3-methyl butyl acetate extracted of Jackfruit with different functional groups in their active sites. Our calculations have demonstrated that such extrapolation schemes significantly overestimate the effective compounds-SWCNT shifts that the O and S atoms were the most active point at indicated structure.
References
- Rahman MA, Nahar N, Mian AJ, Moshiuzzaman M. Food Chem 1999, 65: 91–7.
- De Faria AF, De Rosso VV, Mercadante AZ. J Plant Foods Hum Nutr 2009, 64:108–15.
- Alzamora SM, Salvatori D, Tapia SM, Lopez-Malo A, Welti-Chanes J, Fito P., J Food Eng 2005, 67: 205–14.
- Bayer G, Wense T., Pflugers archive european. J Phys 1936, 237: 417.
- Balasundram N, Sundram K, SammanS Food Chem 2006, 99: 191–203.
- Bandyopadhyay D, Bandyopadhyay A, Das PK, Reiter RJ. J Pineal Res 2002, 33(1):1–7.
- M. Monajjemi, and M. Ahmadianarog, Journal of Computational and Theoretical Nanoscience, 2014, 11(6), 1465-1471.
- A. Tahan and M. Monajjemi, Acta Biotheor 2011, 59: 291–312
- Bhat AV, Pattabiraman TN., J Biosci 1986, 14(4), 351–65.
- M. Monajjemi, S. Afsharnezhad, M.R. Jaafari, T. Abdolahi, A.Nikosade and Monajemi. Russian Journal of physical chemistry A, 2007, 2,1956-1963.
- M. Monajjemi, M. Khaleghian, J Clust Sci., 2011, 22, 673.
- Borrelli F, Izzo AA., The plant kingdom as a source of anti-ulcer remedies. Phytother Res 14(8):581–91.
- H. Yahyaei& M. Monajjemi, Fullerenes, Nanotubes, and Carbon Nanostructures, 2000, 22: 346-361, 2014
- Chandrika UG, Jansz ER, Warnasuriya ND J Sci Food Agric 2005, 85(2):186–90.
- H. Yahyaei , M. Monajjemi , H. Aghaie , and K. Zare, Journal of Computational and Theoretical Nanoscience, 2013, 10(10), 2332-2341.
- Majid Monajjemi, Robert Wayne, Jrand James E. Boggs, Chemical Physics, 2014, 433, 1-11.
- De Faria AF, De Rosso VV, Mercadante AZ J Plant Foods Hum Nutr 2009, 64:108–15.
- Monajjemi M, Ghiasi R, Ketabi S , Journal of Chemical Researchs 2004, 1: 11-1.
- Soong YY, Philip Barlow J., J Agric Food Sci Chem 2004, 88: 411–7.
- T. Ardalan , P. Ardalan& M. Monajjemi, Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 687–708.
- Kagan VE, Kisin ER, Kawai K., Ann N Y Acad Sci 2002, 959: 188–98.
- M.Monajjemi, M.H.Razavian, F.Mollaamin, F.Naderi, B.Honarparvar, Russian Journal of Physical Chemistry A 2008, 82(13), 2277-2285.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J., Int J Biochem Cell Biol 2007, 39: 44–84.
- Kopsell DA, Kopsell DE., Trends Plant Sci 2006, 11: 499–507.
- Umesh JB, PanaskarShrimant N, Bapat VA., Plant Foods Hum Nutr 2010, 65: 99–104.
- BapatVishwas A., Ind Crops Prod 2011, 12(09): 512–8.
- Monajjemi M, Chahkandi B., J. Mol. Struct. (Theochem), 2004, 714: 43.
- Monajjemi M, Ghiasi R, Sadjadi M.A.S, Applied organometallic chemistry 2003, 17(8): 635-640.
- Garci1a-Segovia P, Andres-Bello A, Martinez-Monzo J., J Food Eng 2007, 80: 813–21.
- Madhav Satheesh NV, Shakya A, Shakya P, Singh K, Journal of controlled release., 2009, 140: 2-11.
- Umesh JB, WaghmareShailesh R, Lokhande Vinayak H, PennaSuprasanna, Venkataraman K., Phytochemistry 2001, 11(5): 1571–86.
- Basit and Lacey, A. Basit and L . Lacey, Colonic metabolism of ranitidine: implications for its delivery and absorption, Int. J. Pharm 2001, 2001; 227:157.
- M. Monajjemi , N. Farahani , and F. Mollaamin, Physics and Chemistry of Liquids, 2012, 50 2), 161–172
- Smart JD, Adv Drug Deliv Rev 2005; 57: 1556-68.
- Wallace JL., Physiol Rev 2008, 88:1547–65.
- Stahl W, Sies H., Biochim Biophys Acta 2005, 1740: 101–7.
- Tulyathan V, Tananuwong K, Songjinda P, Jaiboon N., Science Asia 2002, 28:37–41.
- F. Mollaamin, K. Shahani pour, K. Shahani pour, A. R. Ilkhani, Z. Sheckari, and M.Monajjemi, Russian Chemical Bulletin, International Edition, 2012, 61(12), 2193-2198.
- Monajjemi, M., Aghaie, H.,Naderi, F. Biochemistry (Moscow) 2007, 72(6): 799-804.
- Fan J, Watanabe T., J AtherosclerThromb 2003, 10: 63–71.
- Rates SM., Toxicon 2001, 39: 603–13.
- Niva M., Appetite 2007, 48: 384–93.
- Galaverna G, Di Silvestro G, Cassano A, Sforza S, Doceana A, Drioli E, Marchelli R Food Chem 2008, 106: 1021–30.
- Ocloo FCK, Bansa D, Boatin R, Adom T, Agbemavor WS., AgricBiol J N Am , 2010, 1(5): 903–8.
- Vinuda-Martos M, Ruiz-NAvajas Y, Fernandez-Lopez J, Perez-Alvarez JA., Food Sci Food Safety 2010, 9:240–58.
- Roberfroid MB., Br J Nut 2002, 88:133–8.
- Pereira-da-Silva GAN, Moreno F, Marques C, Jamur A, Panunto-Castelo MC., Biochim Biophys Acta 2006, 1(1): 86–94.
- Xu S, Touyz RM., Can J Cardiol 2006, 22(11): 947–51
- Theivasanthi T, Alagar M., Nano Biomed Eng 2011, 3(3):163–8.
- Widmer R, Ziaja I, Grune T., Free Radical Res, 2006, 40(12):1259–68.
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruma OI, Bahorun T., Mutation Res 2005, 579: 200–13.
- Mukprasirt A, Sajjaanantakul K., Intl J Food Sci Technol 2004, 39(3): 271–6.
- Marine FR, Martinez M, Uribesalgo T, Castillo S, Furtos MJ., Food Chem 2001, 78:319–24.
- M. Monajjemi, M. Khosravi, B. Honarparvar , International Journal of Quantum Chemistry, 2011, 111, 2771-2777.
- M. Monajjemi, J.E Boggs, J. Phys. Chem A. 2013, 117, 1670.
- Majid Monajjemi, FatemehMollaamin , TaherehKarimkeshteh , J. Mex. Chem. Soc. 2005, 49(4), 336-340.
- Majid Monajjemi, Chemical Physics, 2013, 425, 29-45.
- Monajjemi, M., Lee, V. S., Khaleghian, M., Honarparvar, B., and Mollaamin, F., J. Phys. Chem. C., 2010, 114: 15315-15330.
- Palou A, Serra F, Pico C., Eur J ClinNutr 2003, 57: 12–7.
- M. Monajjemi, M. Heshmata,H. H. Haeri, Biochemistry (Moscow), 2006, 71, S113-S122.
- M. Monajjemi, M. Heshmat, H. H. Haeri, F. Kaveh, Russian Journal of Physical Chemistry. 2006, 80,1061-1068.
- M. Monajjemi & M. Falahati & F. Mollaamin, Ionics 2013, 19: 155-164
- M.Monajjemi, M.Heshmat, H.Aghaei, R. Ahmadi,K.Zare Bulletin of the Chemical Society of Ethiopia., 2007, 21, 111-116.
- M.Monajjemi, E,Rajaeian, F. Mollamin, F. Naderi, S. Saki, Physics and Chemistry of Liquids., 2008, 46, 299-306.
- GAUSSIAN 03, Revision C.02: M.J. Frisch et al. Gaussian Inc., Wallingford CT, 2004.
- Setiawan B, Sulaeman A, Giraud DW, DriskellJA ., J Food Comp Anal 2001, 14: 169–76.
- Majid Monajjemi • Fatemeh Mollaamin, J Clust Sci., 2012, 23: 259-272.
- F. Mollaamin, Z. Varmaghani , and M. Monajjemi, Physics and Chemistry of Liquids. 2011, 49(3), 318–336
- Vilela L, Carvalho JE., Phytomedicine 2005, 12: 72–7.
- M. Monajjemi, S. Ketabi, M. Hashemian Zadeh, and A. Amiri, Biochemistry (Moscow), 2006, 71(1), S1-S8
- F. Mollaamin, J. Najafpour, S. Ghadami, A. R. Ilkhani, M. S. Akrami, and M. Monajjemi J. Comput. Theor.Nanosci. 2014, 11, 1290-1298.
- M. Monajjem, S. Ketabi, A. Amiri, Russian Journal of Physical Chemistry , 2006, 1, 55-62.
- M. Monajjemi, Struct Chem., 2012, 23., 551-580.
- F. Mollaamin, M. Monajjemi& J. Mehrzad , Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 738-751.
- M. Monajjemi& J. Najafpour, Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 575–594.
- Fatemeh Mollaamin and Majid Monajjemi, Physics and Chemistry of Liquids, 2012, 50(5): 596–604
- Monajjemi,M. Honarparvar, B. H. Haeri, H. Heshmat, M., Russian Journal of Physical Chemistry C., 2006, 80(1): S40-S44.
- Majid Monajjemi and A.Abedi,Research Journal of Chemistry and Environment, 2006, 4,37-41.
- Monajjemi M, Ghiasi R, Abedi A,Russian Journal of Inorganic Chemistry, 2005, 50(3), 382-388.
- M. Monajjemi, and F. Mollaamin , Journal of Computational and Theoretical Nanoscience 2012, 9(12), 2208-2214.
- M.Monajjemi, H. Yamola& F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 595–603.
- M. Monajjemi, H. Baheri, and F. Mollaamin, Journal of Structural Chemistry. 2011, 52(1), 54-59.
- M. Monajjemi , J. Najafpour , & F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 2013, 21: 213–232.
- M. Monajjemi , M. Seyed Hosseini& F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 2013, 21, 381-393.
- M. Monajjemi , A. Sobhanmanesh , & F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 2013, 21, 47-63.
- B. Ghalandari, M. Monajjemi, and F. Mollaamin, Journal of Computational and Theoretical Nanoscience, 2001, 8, 1212-1219.
- M. Monajjemi, M. Seyed Hosseini, M. Mousavi & Z. Jamali, Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 798-808.
- M.Monajjemi, M.T.Baei, F.Mollaamin, Russian Journal of Inorganic Chemistry 2008, 53 (9), 1430-1437.
- M. Monajjemi , R. Faham& F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 2012, 20: 163-169.
- F. Mollaamin and M. Monajjemi, Journal of Computational and Theoretical Nanoscience, 2012, 9(4), 597-601.
- M. Monajjemi, N. Karachi & F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 643-662.
- M. Monajjemi ; H. Chegini ; F. Mollaamin ; P. Farahani,Fullerenes, Nanotubes, and Carbon Nanostructures, 2011, 19: 469-482.
- F. Mollaamin , F. Najafi , M. Khaleghian , B. KhaliliHadad& M Monajjemi , Fullerenes, Nanotubes, and Carbon Nanostructures, 2011, 19: 653-667.

This work is licensed under a Creative Commons Attribution 4.0 International License.









