Synthesis and Spectral Characterization of Lanthanide Complexes Derived from 2-[(4-Bromo-2,6-Dichloro-Phenylimino)-Methyl]-4,6-Diiodo-Phenol
V. R. Rajewar 1, M. K. Dharmale2 , S. R. Pingalkar1
1Department of Chemistry N.E.S.Science College ,Nanded (M.S), India.
2Department of Chemistry Yeshwant Mahavidyalaya ,Nanded (M.S), India.
DOI : http://dx.doi.org/10.13005/ojc/300471
Article Received on :
Article Accepted on :
Article Published : 17 Dec 2014
Bidentate Schiff base; Metal complexes; Thermal analysis; XRD
Download this article as:| Copy the following to cite this article: Rajewar V. R, Dharmale M. K, Pingalkar S. R. Synthesis and Spectral Characterization of Lanthanide Complexes Derived from 2-[(4-Bromo-2,6-Dichloro-Phenylimino)-Methyl]-4,6-Diiodo-Phenol. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Rajewar V. R, Dharmale M. K, Pingalkar S. R. Synthesis and Spectral Characterization of Lanthanide Complexes Derived from 2-[(4-Bromo-2,6-Dichloro-Phenylimino)-Methyl]-4,6-Diiodo-Phenol. Available from: http://www.orientjchem.org/?p=5976 |
Introduction
Schiff bases derived from aromatic amines and aldehydes have a wide variety and an important class of ligands in coordination chemistry and find extensive applications in different fields, e.g., biological, inorganic and analytical chemistry 1.2. Many biologically important Schiff bases have been reported in the literature possessing, antimicrobial, antibacterial, antifungal, anti-inflammatory, antitumor and anti HIV activities 3.4. Schiff bases play important roles in coordination chemistry as they easily form stable complexes with most transition metal ions 5.
The interaction of these donors ligands and metal ions give complexes of different geometries and these complexes are potentially biologically active6. Several research papers reported the synthesis and characterization of transition metal complexes of Schiff bases derived from salicylaldehyde7,8.
Metal complexes with Schiff base ligands containing salicylaldehyde and its derivatives; have been extensively studied. Metal complexes with such ligands are quite common and also reflect their facile synthesis, accessibility of diverse structural modifications and wide applications in different fields, such as catalysis, biological systems and material chemistry9, 10.
Transition metal complexes with Schiff base as ligand have been amongst the widely studied co-ordination compounds in the past few years, since they are found to be widely applicable in many fields such as biochemical, analytical and antimicrobial fields11-15. It is well known from the literature that much work have been done on the synthesis and characterization of this compounds16-18 with Schiff base ligand formed from salicylaldehyde or substituted salicylaldehyde and various aromatic amines19-23.
Salicylic aldehyde is an important intermediate in the manufacture of herbicides and pesticides.28
Also, salicylaldehyde and its derivatives are used for various reactions for the production of polymers and fibers.
Salicylaldehyde is called 2-hydroxybenzaldehyde and ortho-hydroxy benzaldehyde and is an organic compound with the formula C7H6O2. Part of the class hydroxy aromatic aldehydes, aromatic nucleus contains two functional groups: a hydroxyl and aldehyde one. This colorless liquid has a bitter almond odor at higher concentrations The natural oils found in Spiraea [Filipendula (Spiraea) ulmaria (Rosaceae)].
Sweet-smelling flowers containing salicylaldehyde and methyl salicylate, glycosides form. Was also identified as a component of the characteristic flavor of buckwheat.24 Salicilaldehyde is used as an important intermediate in the chemical industry, in medicine. It is used in perfume, fragrances, dyes, pharmaceuticals, etc.25 Salicylaldehyde and its derivatives can be used as preservatives in cosmetic products, 26 fragrances, essential oils in various biological applications.27 Also get in formulation of perfumes and fragrances.
Experimental
All chemicals and solvents are used AR grade. All the metals were used as their chloride salts. UV spectra recorded on UV-vis spectrophotometer 119. Conductance or metal complex was determined in DMSO on conductivity meter quiptronics model NO-EQ665. Melting points were recorded on in recorded by open capillary method and are uncorreded. H1-NMR spectra or a Schiff base and its metal complex recorded on Brukcer 300 MHz spectrometer in DMSO. Elemental analysis was carried out on Eager 350 analyser. Magnetic measurement were done on solid complexes using Guoy method. Powder XRD pattern of complexes are recorded Philips Analytical XRD B.V. at CFC Shivaji University Kolhapur.
Synthesis of Ligand
Synthesis of 2-[(4-Bromo-2,6-Dichloro-Phenylimino)-Methyl]-4,6-Diiodo-Phenol (BDPDP) Schiff Base
Schiff base ligand were synthesized by refluxing of 3,5 diiodosalicylaldehyde (0.01M) and 4-bromo-2,6-dichlorobenzenamine (0.01 M) in 50m1 ethanol on water bath for 4-5 hours in presence of 2-3 drops of glacial acetic acid. The reaction mixture was kept for overnight, where yellow color precipitate was obtained. It was filtered by whatmann paper, washed with distilled water then alcohol, dried in vacuum dessicator. Pure Schiff base was recrystallized from ethanol. The purity of ligand was checked by TLC.
 |
Scheme 1 Click here to View scheme |
Synthesis of Metal Complexes
The ethanolic solution of Metal chloride (0.01M) was added to hot ethanolic solution of BDPDP (0.02 M) La(III), Pr (III), Tb(III) ,Sm(III) and (0.01 M) in Nd(III) complexes drop wise with constant stirring. PH of the reaction mixture was adjusted to 7 -7.2 with alcoholic ammonia solution. Resulting reaction mixture refluxed for 5 to 6 hours on water bath.
Colored complexes was allowed to digest and collected by filtration. Then washed with sufficient quantity of distilled water and little hot ethanol to apparent dryness and dried in vacuum desiccators.
Results and Discussion
Physical and Analytical Parameters
Empirical formulae of the complexes were deduced on the basis of elemental analysis, metal ligand ratio and thermal analysis (table No. 1.1 and 1.2). Complexes possess different colors, metal complexes of ligand BDPDP are insoluble in common organic solvents, dissolve freely in DMSO/DMF High melting points of complexes suggest that complexes are stable at normal temperatures29. Molar conductivity (lm 71 to 82 W-1cm2mol-l) reveals electrolytic nature of the complexes30 except Neodymium metal ion complex (lm 14W-1 cm2mol-l) reveals nonelectrolytic nature of the complex.(table 1.1).
Table 1.1: Physical and analytical data of BDPDP metal complexes.
|
Compound |
F.W. |
Yield |
M.L ratio |
M.P. decom. Temp °C |
Color
|
Molar Conductance W-1 cm2 mol-1 |
%of Cl cal. (obs) |
Magnetic Moment |
|
BDPDP(L) |
596.81 |
67 % |
—- |
1900C |
Yellow |
—- |
—- |
—- |
|
[La(L)22H2O]Cl |
1404.01 |
58 % |
1:2 |
>270 0C |
Muddy Yellow |
66 |
12.63 (12.59) |
Dimagnetc |
|
[Pr(L)22H2O]Cl |
1408.03 |
62 % |
1:2 |
>270 0C |
Brown |
71 |
12.59 (12.57) |
Paramagnetic |
|
[Nd(L)12H2O2Cl] |
847.99 |
58 % |
1:1 |
>270 0C |
Grey |
14 |
16.72 (16.74) |
Paramagnetic |
|
[Tb(L)22H2O]Cl |
1426.05 |
51 % |
1:2 |
>270 0C |
Dark Yellow |
78 |
12.43 (12.41) |
Paramagnetic |
|
[Sm(L)22H2O]Cl |
1412.33 |
61 % |
1:2 |
>270 0C |
Faint Yellow |
64 |
12.52 (12.48) |
Paramagnetic |
Table 1.2: Percent C, H, N,O and metal ion in BDPDP metal complexes.
| Compound |
Empirical Formula |
%C obs (calcd.) | %H obs (calcd.) | %N obs (calcd.) | %O obs (calcd.) | % I obs (calcd.) | % Br obs (calcd.) | %M obs (calcd.) |
| BDPDP(L) | C13H6BrCl2I2NO |
26.16 (26.12) |
1.01 (0.92) |
2.35 (2.24) |
2.68 (2.46) |
42.53 (42.42) |
13.39 (13.12) |
—- |
| [La(L)22H2O]Cl | C26H16Br2Cl5I4LaN2O4 |
22.24 (22.14) |
1.15 (1.11) |
2.00 (1.98) |
4.56 (4.31) |
36.15 (36.23) |
11.38 (11.23) |
9.89 (9.78) |
| [Pr(L)22H2O]Cl | C26H18Br2Cl5I4N2O4Pr |
22.18 (22.09) |
1.29 (1.14) |
4.55 (4.34) |
4.55 (4.43) |
36.05 (35.95) |
11.35 (11.27) |
10.01 (9.67) |
| [Nd(L)12H2O2Cl] | C13H10BrCl4I2NNdO3 |
18.41 (18.31) |
1.19 (1.04) |
1.65 (1.45) |
5.66 (5.45) |
29.93 (29.84) |
9.42 (9.31) |
17.01 (16.47) |
| [Tb(L)22H2O]Cl | C26H18Br2Cl5I4N2O4Tb |
21,90 (21.87) |
1.27 (1.16) |
1.96 (1.89) |
4.49 (4.38) |
35.60 (35.49) |
11.21 (11.14) |
11.14 (11.03) |
| [Sm(L)22H2O]Cl | C26H16Br2Cl5I4N2O4Sm |
22.06 (21.89) |
1.14 (1.08) |
1.98 (1.78) |
4.52 (4.43) |
35.86 (35.73) |
11.29 (11.18) |
10.62 (10.54) |
Electronic Spectra
Plots of UV-Visible spectra of ligand BDPDP and its metal complexes were recorded on UV-Visible spectrophotometer 119-Pc based instrument are presented in figure 6.1, 6.2, 6.3 , 6.4,6.5 and 6.6. Ligand (BDPDP) shows strong absorption band at 34160 cm-1 assigned for π – π* transition. Absorption bands and corresponding transition are given in the table No. 1.3.
Table 1.3: Electronic spectral data of BDPDP complexes.
|
Ligand / Complex |
Absorbance nm |
n/cm-1 |
Transition |
| BDPDP(L) |
293 |
34160 |
π – π* |
| [La(L)22H2O]Cl3 |
261 |
38314 |
π – π* |
|
431 |
23201 |
LMCT |
|
|
261 |
38314 |
π – π* |
|
431 |
23201 |
LMCT |
|
|
259 |
38610 |
π – π* |
|
431 |
23201 |
LMCT |
|
|
261 |
38314 |
π- π* |
|
429 |
23310 |
LMCT |
|
|
261 |
38314 |
π- π* |
|
429 |
23310 |
LMCT |
The UV electronic spectra of La(III), Pr (III), Nd(III), Tb(III) and Sm(III)complexes have indicates absorption bands at 23201 cm-1, 23201 cm-1, 23201 cm-1,23310 cm-1 and 23310 cm-1 assigned as charge transfer31,32.
Infrared Spectra
Determination of coordinating atoms in the complex is made on the basis of comparison of IR spectra of the ligand and their metal complexes. Significant changes in wave numbers of the coordinating atoms involved in coordination are summarized in the table No. 1.4.
IR Spectral Study of BDPDP Ligand
Ligand BDPDP contains phenolic –OH and azomethine group. In spectra of ligand exhibits strong n (O-H) stretching at 3447 cm-1, corresponding to n (OH) of phenol. The band at 1204cm-1 is due to presence of n (C-O) group. It also indicate n (C=N) stretching frequency at 1616cm-1. On complexation significant changes in wave numbers are observed.
IR Spectral Study of La(III) Complex
The IR spectra of Metal complexes is compared with IR spectra of ligand (BDPDP), there are certain shifts in the bands. In complex deprotonation of —OH in phenolic group and indicating involvement of phenolic group in coordination 33. The band stretching vibration in ligand due to phenolic n(OH) group observed at 3447cm-1, Which is disappear in complex.
Besides, ligand exhibits stretching of n (C=N) stretching at 1616cm-1 which on complexation shifted to lower wave number at 1596-1631cm-1suggesting that azomethine nitrogen are involved in coordination34′ 35 .A new broad band at 3134-3477cm-1 suggested the presence of coordinated water molecule36.
The appearance of new bands in the spectra of metal ion complex at 432-441cm-1 and 505-520 cm-1 due to new bonding i.e. n (M-N) and n (M- O)37,38.
Thus the ligand BDPDP exhibits uninegative bidentate behaviour and coordinates to the metal ion through azomethine nitrogen and phenoxide oxygen for La(III), Pr (III), Tb(III) and Sm(III)complexes, Nd(III) complex behave neutral in nature.
Table No 1.4 Infrared spectral data of the ligand (DPMDI) and their La(III), Pr (III), Nd(III), Tb(III) and Sm(III) metal complexes
|
Compound |
n(CH=N) |
n(C-O) |
n(M-O) |
n(M-N) |
n(H2O) Rocking |
n(H2O) |
n(OH) |
|
BDPDP(L) |
1616 |
1204 |
—- |
—- |
—- |
—- |
3447 |
|
[La(L)22H2O]Cl |
1596 |
1212 |
520 |
432 |
853 |
3134 |
—- |
|
[Pr(L)22H2O]Cl |
1627 |
1209 |
505 |
432 |
860 |
3477 |
—- |
|
[Nd(L)12H2O2Cl] |
1629 |
1211 |
507 |
435 |
869 |
3448 |
—- |
|
[Tb(L)22H2O]Cl |
1627 |
1211 |
507 |
441 |
889 |
3437 |
—- |
|
[Sm(L)22H2O]Cl |
1631 |
1211 |
509 |
435 |
867 |
3473 |
—- |
1HNMR Spectra
1H NMR spectral studies of ligand BDPDP indicated signals at d 6.9- 7.2 ppm corresponding to aromatic protons (m, 4H, Ar-H) and at d 7.9 ppm due to azomethine group (CH=N). A strong signal at d 13.3 ppm assignable (S, 1H) due to phenolic OH group (fig. 1.1).
In the metal complex signal corresponding to phenolic OH group at d 13.3 ppm has disappeared39-41 (fig.1.2) may be attributed to deprotonation of —OH group on involvement of via -OH in bonding. A new peak due to presence of coordinated water at d 2.5 ppm is observed in metal complex42 .The shift in azomethine group from d 7.9 ppm to d 9.6 ppm indicate coordination through water molucule azomethine group.
Thus, BDPDP.molecule seems to be coordinated to the metal through phenoxide oxygen and azomethine group in Pr (III) metal complex.
 |
Figure6.13: 1H NMR ligand BDPDP Click here to View figure |
 |
Figure1.2: 1H NMR Metal complex Click here to View figure |
Thermal Study
Nd(III), Pr(III) and Tb(III) complexes were studied by therrnogravimetric analysis from ambident temperature to 1000°C in nitrogen atomosphere. The range of temperature, experimental and calculated mass losses of the decomposition reaction are given in the table No. 1.5. TGA/DSC scans are depicted in figures 1.3, 1.4 and 1.5.
Thermal Study of [Tb(L)22H2O]Cl
The thermogram of Tb(III) complex shows weight loss 2.44% corresponding to two coordinated water molecule in the range from room temperature to 140°C. Decomposition reaction corresponds to an experimental mass 2.42 % occurs in the temperature range 140°C- 280°C attributed loss of one lattice chlorine 43. part of the complex.
In the temperature range 280°C-310°C Four iodine part is lost and this loss corresponds to 38.04%. As the temperature increases to 310-600°C there is of 18.16 % indicating loss of four chloride,two bromine , part of metal complex. From 600-8000C loss of organic moiety which is 28.12 %.Finally 800°C-1000°C residue is obtained corresponding to Tb2O3 as stable residue 44 12.44%.
Thermal Study of [Pr(L)22H2O]Cl
The TGA of Pr(III) complex indicates loss in weight in the range from room temperature to 160°C corresponding to 2.32 % indicates the loss of coordinated water-molecule.
Decomposition beyond this temperature in the range 160°C -390°C corresponds to mass loss of 61.32 % in the TG curve assigned to expulsion of one lattice chloride molecules four iodine, four chloride , two bromine part of the complex. The decomposition occurs in the temperature 390°C -800°C indicates the loss of Organic Moiety 27.68 %. Further at 800°C-1000°C losses of 11.87 % were occurs indicating presence of thermally stable residual metal oxide45.
Thermal Study of [Nd(L)12H2O2Cl]
TGA of Nd (III) complex shows weight loss corresponding to mass loss 4.25 %. This loss corresponds to loss of coordinated water molecule in the range from room temperature to 160°C46. Further decomposition at 160°C-430°Closs of 36.71 % was occurs indicating loss of Two iodine, One bromine of the complex. From 430-8000C loss of four chlorine , Organic moiety which is 33.64 %.The end product of decomposition is formation of Nd2O3 weight corresponds to 19.58 percent which is equal to theoretical value 20.09.
Table-1.5 Thermal Analysis data for metal complexes
| Complex | Decomposition Temp°C | Lost fragment |
Weight loss % |
|
|
Experimental |
Theoretical |
|||
| [Tb(L)22H2O]Cl | 140oC | two coordinate water molecule |
2.44 |
2.56 |
| 140-280 oC | one lattice chlorine |
2.42 |
2.53 |
|
| 280-310 oC | Four iodine |
38.04 |
36.22 |
|
| 310-600 oC | Four chlorine two bromine |
18.16 |
21.53 |
|
| 600-800 oC | Organic moiety |
28.12 |
27.23 |
|
| 800-1000 oC | Metal oxide |
12.44 |
13.04 |
|
| [Pr(L)22H2O]Cl | 160oC | two coordinated Water molecules |
2.32 |
2.60 |
| 160-390 oC | one lattice chloride molecules four iodine, four chloride , two bromine |
61.32 |
61.02 |
|
| 390-800 oC | Organic Moiety |
27.68 |
26.64 |
|
| 800-1000 oC | Metal oxide |
11.87 |
11.91 |
|
| [Nd(L)12H2O2Cl] | 160 oC | coordinated two water molecules |
4.25 |
4.30 |
| 160-430 oC | Two iodine, One bromine |
36.71 |
39.89 |
|
| 430-800 oC | four chlorine , Organic moiety |
33.64 |
32.60 |
|
| 800-1000 oC | Metal oxide |
19.58 |
20.09 |
|
![Figure 1.3: TG/DSC [Tb(L)22H2O]Cl](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_SYNTH_Rajew_Fig1.3-150x150.jpg) |
Figure1.3: TG/DSC [Tb(L)22H2O]Cl Click here to View figure |
![Figure 1.4: TG/DSC [Nd(L)12H2O2Cl]](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_SYNTH_Rajew_Fig1.4-150x150.jpg) |
Figure1.4: TG/DSC [Nd(L)12H2O2Cl] Click here to View figure |
![Figure 1.5: TG/DSC [Pr(L)22H2O]Cl](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_SYNTH_Rajew_Fig1.5-150x150.jpg) |
Figure1.5: TG/DSC [Pr(L)22H2O]Cl |
Powder X-Ray Studies
X-ray diffractograms of the metal complexes were recorded in the 2q range from 10-90° at a wave length of 1.5405 A°and using Cu Ka radiation source. Results of miller indices, lattice parameters and unit cell volume are computed from programmer. Data has been summarized in the following tables.
[Nd(L)12H2O2Cl]
Crystal system: Monoclinic Lattice Type: P
Lattice Parameter: a= 9.53034 b= 8.00951 c= 7.79302 A0
Lattice Parameter: Alpha= 90.000 Beta= 121.572 Gama=90.000
| h k l |
2θ (cal ) |
2θ (Obs) |
d(cal) |
d (obs) |
| -1 0 1 |
6.00366 |
6.01408 |
7.36480 |
7.34506 |
| -1 1 0 |
7.76464 |
7.75704 |
5.70152 |
5.70286 |
| -2 0 0 |
10.94017 |
10.92606 |
4.05882 |
4.06188 |
| -2 1 2 |
13.31070 |
13.30282 |
3.34576 |
3.34628 |
| -2 2 1 |
14.58616 |
14.57042 |
3.05874 |
3.06078 |
| -4 1 0 |
23.05168 |
23.04753 |
1.96725 |
1.96712 |
| 3 0 2 |
26.85357 |
26.85035 |
1.70529 |
1.70514 |
| -4 2 5 |
32.39762 |
32.39613 |
1.43768 |
1.43751 |
| -1 5 3 |
34.86598 |
34.85211 |
1.34748 |
1.34775 |
| -2 6 1 |
36.83277 |
36.83274 |
1.28494 |
1.28477 |
| -2 1 6 |
38.73699 |
38.73415 |
1.23101 |
1.23093 |
| -4 3 6 |
41.28170 |
41.26937 |
1.16754 |
1.16769 |
| -4 6 3 |
42.69762 |
42.69542 |
1.13592 |
1.13584 |
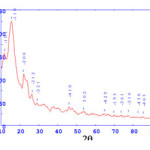 |
Figure 1 Click here to View figure |
The cell data and crystal parameters of [Nd(L)12H2O2Cl] complex is given in the tables indicates that complex have monoclinic crystal system47, with lattice type-P.
[Pr(L)22H2O]Cl
Crystal system: Monoclinic Lattice Type: P
Lattice Parameter: a= 19.06907 b= 4.64343 c= 7.04556 A0
Lattice Parameter: Alpha= 90.000 Beta= 108.476 Gama=90.000
|
h k l |
2θ (cal ) |
2θ (Obs) |
d(cal) |
d (obs) |
| -2 0 1 |
6.87288 |
6.88556 |
1.27114 |
6.43274 |
|
1 0 1 |
7.75612 |
7.75704 |
5.70775 |
5.71298 |
|
2 0 1 |
9.41625 |
9.42077 |
4.70827 |
4.71002 |
| -1 1 1 |
11.46505 |
11.48063 |
3.87533 |
3.87282 |
|
1 1 1 |
12.34822 |
12.35211 |
3.60201 |
3.60321 |
|
1 0 2 |
14.32149 |
14.33275 |
3.11405 |
3.11337 |
| -7 0 2 |
18.28842 |
18.29401 |
2.45474 |
2.45506 |
| 0 2 2 |
23.82950 |
23.83979 |
1.90660 |
1.90644 |
| 8 1 2 |
29.77117 |
29.78169 |
1.55134 |
1.55123 |
| 0 3 3 |
37.30260 |
37.30810 |
1.27107 |
1.27114 |
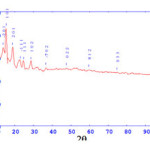 |
Figure 2 Click here to View figure |
Cell data and crystal lattice parameters of [Pr(L)22H2O]Cl complex attributed to monoclinic crystal system48, with lattice type-P.
Proposed Structures of the Chelates
Based on above result probable structure have been proposed
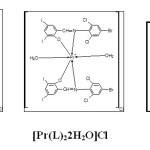 |
Figure 3 Click here to View figure |
References
- Raman N, Muthuraj V, Ravichandran S, Kulandaisamy A., Synthesis, characterisation and electrochemical behaviour of Cu(II), Co(II), Ni(II) and Zn(II) complexes derived from acetylacetone and p-anisidine and their antimicrobial activity. Journal of Chemical Sciences, 2003, 115(3): 161–167.
- Hughes, M. N., “The inorganic chemistry of biological Processes”, 2nd ed., Wiley, New York, 1984.
- Pandeya S.N., Sriram D., Nath G. and Clercq E. De, Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis- Schiff bases of isatin and their derivatives,Pharm. Acta Helv.1999, 74: 11.
- Pandeya S.N., Sriram D., Nath G. and Clercq E. de, Synthesis, antibacterial, antifungal and anti- HIV evaluation of Schiff and Mannich bases of isatin and its derivatives with triazole, Arzneimittel Forsch, , 2000, 50: 55
- Spinu C., Pleniceanu M. and Tigae C., Biologically Active Transition Metal Chelates with a 2-Thiophenecarboxaldehyde -Derived Schiff Base: Synthesis, Characteri-zation, and Antibacterial Properties, Turk. J. Chem., 2008, 32: 487.
- Harlal S. and Varshney A. K., “Synthetic structural and biochemical studies of organotin (IV) with Schiff bases having nitrogen and sulfur donor ligands”, bioinorganic chemistry and applications, Article ID 23245:1-7, 2006.
- Chaurasia M. R., Miss Prema S. and Singh N. K., “Mixed ligand complexes of N- 6-methyl benzothiazol-2-yl-salicylaldimine and 2- methylbenzimidazole with Cu(II), Ni(II), Co(II), Mn(II), VO(II), Zn(II), Cd(II) and Hg(II), 1982, 74, 123-128.
- Shayma A. Shaker, Yang F., Abbas A. S., Synthesis and Characterization of Mixed Ligand Complexes of 8-Hydroxy-quinoline and o-hydroxybenz-ylidene-1-phenyl-2,3-dimethyl – 4-amino-3-pyrazolin -5-on with Fe(II), Co(II), Ni(II) and Cu(II) ions, European Journal of Scientific Research, 2009, 33 (4), 702-709.
- Iqbal, J., Tirmizi, S. A., and Watto, F. H., Biological properties of chloro-salicylidene aniline and its complexes with Co(II) and Ni(II). Turk J. Biol., 2006, 30, 1-4.
- Bagihalli, G. B., Patil, S. A., and Badami, P. S., Synthesis, physicochemical investigation and biological studies of Zn(II) complexes with 1,2,4-triazole Schiff bases. J. Iran Chem. Soc., 2009, 6, 259-270.
- N.L.Wengnack, H.M. Hoard and F. Rusnak, J. Am. Chem., 1999, 121, 9748.
- N.H. Bhausar, B.D. Mistry and K.R. Desai, Asian J. Chem., 1999, 11, 65.
- M.R. Maurya, Co.ord. Chem. Rev., 2003, 237,163.
- G.D. Whitener, J.R. Hagadorn and Amold, J, Chem., Soc. Dalton Trans, 1999, 8, 1249.
- M.M. Patel and K.C. Patel, J. Indian Chem. Soc., 1997, 1, 74.
- A.K. Usha and S. Chandra, Synth. React. Inorg. Met.-Org. Chem., 1992, 2, 1565.
- B.S. Garg and L. Kapur, Inorg.Chim.Acta, 1990, 173, 223.
- Z.H. Chohan and S. Kausar, Chem. Pharm. Bull., (Japan) 1993, 41, 951.
- R.H.Holm, G.W. Everett and A. Chakravorty, Prog. Inorg. Chem., 1966, 7, 83.
- L. Sacconi, Coord. Chem. Rev., 1966, 1, 126, 192.
- M. Akbar Ali and S. E. Livingstone, Coord. Chem. Rev., 1970, 3,101.
- A.V. Ablov, N.I. Belichuk and L.N. Nazhelskaya, Russ. J. Inorg. Chem., 1972, 17, 226.
- M.D. Hobday and T.D. Smith., Coord. Chem. Rev., 1973, 9, 311.
- Janeš D.; Kreft S., “Salicylaldehyde is a characteristic aroma component of buckwheat groats”, Food Chemistry, 2008, 109(2), 293–298.
- Simmonds J., Robinson G. K., “Formation of Benzaldehyde by Pseudomonas putida ATCC 12633”, Appl. Microbial Biotechnol, 1998, 50, 353-358.
- Genta M.T., Villa C., Mariani E., Loupy A., Petit A., Rizzetto R., Mascarotti A., Morini F., Ferro M., “ Microwave-assisted preparation of cyclic ketals from a cineole ketone as potential cosmetic ingredients: solvent-free synthesis, odour evaluation, in vitro cytotoxicity and antimicrobial assays”, Int. J. Pharm., 2002, 231, 11-20.
- Mounika K, Anupama B., Pragathi J.,Gyanakumari C., “Synthesis¸ Characterization and Biological Activity of a Schiff Base Derived from 3-Ethoxy Salicylaldehyde and 2-Amino Benzoic acid and its Transition Metal Complexes”, J. Sci. Res., 2010, 2(3)., 513-524.
- Yildiz M., Dulger B., Koyuncu S.Y., Yapici B.M., “Synthesis and antimicrobial activity of bis(imido) Schiff bases derived from thiosemicarbazide with some 2-hydroxyaldehydes and metal complexes”, J. Indian Chem. Soc, 2004, 81, 7-12.
- Rosu, T., Pahontu, E., Maxim, C., Georgescu, R., Stanica, N., Gulea, A., Polyhedron, 2011, 30(1), 154.
- Reddy, P.R. and Reddy, A.M., I.J.Chem., 2002, 41A, 2083.
- Pandey, R.N. and Nag, A.K., Rasayan J. Chem., 2009, 2(4), p. 990.
- Abdullah, B.H., Asian Journal of Chemistry, 2007, 19(5), 3903.
- Thirumagal, B., Malik, S. and Balasubramaniam, A., The Pharmacist, 2008, 3(1).
- Kriza, A., Parnau, C., Popu. N. and Rosu, T., Analele Universitatii din Bucuresti Chimie, Anul XIII (Serienoua), 2004, 1-2, 179.
- Subudhi, B.B., Panda, P.K., Bhatta, D., Jena, A., Iranian Journal of Pharmaceutical Sciences Spring, 2009, 5(2), 83.
- Canpolat, E. and Kaya, M., Transition Metal Chemistry, 2004, 29, p.550.
- Malik, S., Ghosh, S. and Jain, B., Arch. Apll. Sci. Res., 2(2), 2010, p. 304.
- Mruthyunjayaswamy, B.H.M., Ijare, O.B. and Jadegoud, Y., J. Braz. Chem. Soc., 2005, 16(4), 783.
- Nakamoto, K., Infrared spectra of inorganic and coordination compounds. John Wiely, New York, 1968.
- Halli, M.B., Qureshi, Z.S., Vittal, P., Jumanal, B.N. and Patil, V.B., J. Ind. Council. Chem., 2008, 25(1), 1 .
- Reddy, V., Patil, N. and Patil, B.R., J. Ind. Council. Chem., 2006, 23(2), 1.
- Pingalkar, S.R. and Deshpande, M.N., Orient. J. Chem., 2007, 23(1), 265.
- Lakshmi, B., Shivananda, K.N., Gouda, A.P., Reddy, K., Rama, K. and Mahendra, K.N., Bull Korean Chem. Soc., 2011, 32(5).
- Nishide, T., Journal of Materials Sciences, 2000, 35, 465.
- Yaul, S.R., Yaul, A.R., Pethe, G.B. and Aswar, A.S., American-Eurasian Journal of Scientific Research, 2009, 4(4), 229.
- RevanaSiddapa, H.D., Vijaya, B., Shiva Kiimar, L. and Pmsad, K.S., World Journal of Chemistry 2010, 5(1), p.18.
- Gigant, K., Rammal, A. and Henry, M., J. Am. Chem. Soc., 2001, 123, 11632.
- Gendler, S., Segal, S., Goldberg, I., Goldschmidt, Z. and Kol, M., Inorg. Chem., 2006, 45(12), 4783.

This work is licensed under a Creative Commons Attribution 4.0 International License.









