Synthesis and Evaluation of Some 2–Aryl–3–[Substituted Pyridin–2–Yl]-Amino/Methylamino Thiazolidin-4-Ones
Department of Chemistry, Gandhi Faiz-e-Aam (P. G.) College, Shahjahanpur-242001, U. P., India.
Corresponding Author E-mail: apkahkashanbegum@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340647
Article Received on : 23-11-2018
Article Accepted on : 20-12-2018
Article Published : 14 Dec 2018
A series of compounds incorporating thiazolidinone moiety has been synthesized and screened for their antifungal activity. 2-Aryl-3-[substituted pyridin-2-yl]-amino/methylamino thiazolidin-4-ones have been synthesized by cyclocondensation of [substituted pyridin-2-yl]- araldehydehydrazone and N-Methyl [substituted pyridin-2-yl]-araldehydehydrazone with mercapto acetic acid in dioxane. The initial reactants required for the synthesis were obtained by refluxing 2-hydrazino substituted pyridine and 2-[N-methylhydrazino]-substituted pyridine with different substituted aldehydes. These newly synthesized compounds were then screened for their fungicidal activity against Rhizoctina solani and Fusarium oxysporum. Structures of all these compounds were confirmed by 1H NMR, IR and mass spectrum analysis. Some compounds exhibited excellent fungicidal properties.
KEYWORDS:Fungicidal Activity; Fusarium Oxysporum; Rhizoctina Solani; Thiazolidinones
Download this article as:| Copy the following to cite this article: Begum K. Synthesis and Evaluation of Some 2–Aryl–3–[Substituted Pyridin–2–Yl]-Amino/Methylamino Thiazolidin-4-Ones. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Begum K. Synthesis and Evaluation of Some 2–Aryl–3–[Substituted Pyridin–2–Yl]-Amino/Methylamino Thiazolidin-4-Ones. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=53884 |
Introduction
The pyridine ring is associated with diverse biological activities viz: antibacterial, herbicidal, fungicidal and plant growth regulators.1-7 Union of pyridine ring with N-containing heterocycles provide compounds of enhanced herbicidal activity.8 The investigations of new pyrazine and pyridine derivatives showing antimicrobial activities have been carried out. They appeared to be of elevated activity towards the aerobes.9 At 100 mgL-1, bioassays indicated that some multi-substituted pyridine derivatives have good herbicidal activity on the roots of oilseed rape and barnyard grass.10
The thiazolidine ring by virtue of presence of toxophoric – N=C-S linkage and a cyclic >C=O function in the five membered ring is associated with diverse biological activities.11-16 Reports pertaining to the pesticidal activities to the ring have also been reported.17-19 Coumarin, thiazole and their respective derivatives have been reported to exhibit significant biological activity and are used as pharmaceuticals.20,21 They are capable of imparting antimicrobial properties.22 Amino and urea substituted thiazoles exhibited in vivo activity on duckweed (Lemna paucicostata).23
Keeping all the above facts it was worth interesting to synthesize compounds by incorporating pyridine ring with thiazolidinone ring in view to obtain the compound of better bioactivity.
Experimental
All the chemicals used were of analytical grade either BDH or E. Merck or Fluka and were used as received. The solvents were used after distillation. Melting points were taken in open glass capillaries and were uncorrected. IR spectra were recorded in KBr on a Perkin Elmer 881 spectrophotometer. 1HNMR spectra were recorded on a Perkin-Elmer R-32 [90 MHz] spectrometer in CDCl3 or DMSO-d6.
These analyses were carried out at RSIC, CDRI, Lucknow and at BHU Varanasi. The antifungal activities of the synthesized compound were evaluated against Rhizoctina solani and Fusarium oxysporum by filter paper disc method at 100 ppm and 10 ppm concentrations. The standard fungicide carbendazim was also tested under similar conditions for comparison.
Synthesis 2-Aryl-3- [substituted pyridin-2-yl]- amino thiazolidin-4-one (II)
The synthesis of this compound involves two steps. In step-I substituted pyridine hydrazide reacts with different substituted benzaldehyde in equimolar ratio to form intermediate hydrazones (I) which in step-II undergoes cyclocondensation with mercapto acetic acid to obtain the desired product. (II).
Step I: N-[substituted pyridine-2-yl] araldehyde hydrazone (I)
A mixture of 2-hydrazino-5-triflouromethyl pyridine (1.77g; 0.01M) and 2,4-dichlorobenzaldehyde (1.75g; 0.01M) was refluxed in methanol for 1 hour. The reaction mixture was cooled and poured into ice-cold water. The compound (I) thus separated out was filtered, washed and crystallized from aqueous ethanol.
Yield: 2.4g (72%); M.P.: 196°C
Analysis for C13H8F3Cl2 (Found): C = 46.00; H = 2.48; N = 12.30
(Calculated): C = 46.71; H = 2.39; N = 12.51
1R (KBr) VMAX cm-1: 3205 (-NH); 1622 (C=N); 1555,1511, 1474 (C=C); 750 (C-Cl)
Other compounds of this type were synthesized similarly by taking aldehydes of different R1.
R‘ = H (1.04g)
R‘ = 2-Cl (1.39g)
R‘ = 4-Cl (1.39g)
R‘ = 2-OH (1.21g)
R‘ = 4-OH (1.21g)
R‘ = 4-F (1.23g)
R‘ = 4-CH3 (1.85g)
R‘ = 4-OCH3 (2.01g)
R‘ = 3,4- (OCH3)2 (2.32g)
R‘ = 3-OCH3, 4-OH (1.52g)
R‘ = 3-NO2 (1.60g)
R‘ = 4-N (CH3)2 (1.58g)
R‘ = 2-COOH (1.50g)
The analytical data of these compounds are recorded in Table 1.
Table 1: Analytical data of synthesized compounds (I a ̶ I m).
| Compd No. | R | R‘ | Molecular formula (Formula weight) | Yield% | M.P.(°C) | C | H | N | |||
| Exp. | Cal. | Exp. | Cal. | Exp. | Cal. | ||||||
| Ia | 5-CF3 | H | C13H10N3F3(265) | 75 | 192 | 58.60 | (58.87) | 3.80 | (3.77) | 15.93 | (15.85) |
| Ib | “ | 2-Cl | C13H9N3F3Cl (299.5) | 72 | 163 | 52.00 | (52.09) | 3.07 | (3.00) | 14.22 | (14.02) |
| Ic | “ | 4-Cl | C13H9N3F3Cl (299.5) | 69 | 190 | 51.96 | (52.09) | 2.87 | (3.00) | 14.19 | (14.02) |
| Id | “ | 2-OH | C13H10N3F3O (281) | 65 | 199 | 55.60 | (55.57) | 3.31 | (3.20) | 14.85 | (14.95) |
| Ie | “ | 4-OH | C13H10N3F3O (281) | 68 | 205 | 55.62 | (55.51) | 3.10 | (3.20) | 14.73 | (14.95) |
| If | “ | 4-F | C13H10N3F3O (281) | 64 | 215 | 55.20 | (55.13) | 3.20 | (3.18) | 19.81 | (19.78) |
| Ig | “ | 4-Me | C14H12N3F3 (279) | 70 | 143 | 60.00 | (60.22) | 3.15 | (3.02) | 15.25 | (15.05) |
| Ih | “ | 4-OMe | C14H12N3F3 (279) | 68 | 165 | 56.73 | (56.95) | 3.08 | (3.05) | 14.00 | (14.24) |
| Ii | “ | 3,4-(OMe)2 | C15H14N3F3O (325) | 72 | 130 | 55.40 | (55.38) | 4.25 | (4.31) | 12.80 | (12.92) |
| Ij | “ | 3-OMe,4-OH | C14H12N3F3O2 (311) | 67 | 143 | 54.00 | (54.02) | 3.75 | (3.86) | 13.26 | (13.56) |
| Ik | “ | 3-NO2 | C13H9N4F3O2 (310) | 64 | 187 | 50.12 | (50.32) | 2.80 | (2.90) | 18.13 | (18.06) |
| Il | “ | 4-N(Me)2 | C15H15N4F3 (308) | 65 | 204 | 58.21 | (58.44) | 4.95 | (4.87) | 14.70 | (14.84) |
| Im | “ | 2-COOH | C14H10N3F3O2 (309) | 68 | 172 | 54.21 | (54.37) | 3.03 | (3.24) | 13.40 | (13.59) |
Step-II: 2-Aryl-3- [substituted pyridin-2-yl]- amino thiazolidin-4-one (II)
A mixture of 5-[triflouromethylpyridin-2-yl]- 2,4-dichlorobenzaldehyde hydrazone (3.34g, 0.01M) and mercapto acetic (0.92g, 0.01M) was refluxed for 5 hours in dioxane (30ml). Excess of solvent was removed and the residue was poured into ice-cold water and neutralized by sodium bicarbonate solution. The compound thus obtained was filtered, washed, dried and recrystallized from aqueous ethanol.
Yield: 2.74g (72%); M.P.: 185°C
Analysis for C15H10ON3SF3Cl2) (Found): C = 44.00; H = 2.36; N = 10.01
(Calculated): C = 44.12; H = 2.45; N = 10.29
IR (KBr)cm-1: 3194(NH); 1678(C=O);
1591,1552, 1477 (C=C)
1240, 1121 (C-S-C); 751 (C-Cl)
1H NMR (CDCl3) δ ppm: 2.31 (s, 1H, N-CH-S)
3.60 (s, 2H, O=C˗CH-CH2-S)
7.00 – 7.80 (m, 6H, Ar-H)
8.20 (s, 1H, NH)
Other compounds of the series were prepared similarly using different substituents R‘.
(IIa) R‘ = H (IIe) R‘ = 4-OH (IIi) R‘ = 3,4-(OMe)2
(IIb) R‘ = 2-Cl (IIf) R‘ = 4F (IIj) R‘ = 3-OCH3-4-OH
(IIc) R‘ = 4-Cl (IIg) R‘ = 4-Me (IIk) R‘ = 3-NO2
(IId) R‘ = 2-OH (IIh) R‘ = 4-OMe (IIl) R‘ = 4-N (Me)2
(IIm) R‘ = 2-COOH
The analytical data of these compounds are recorded in Table 2.
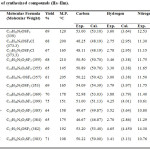 |
Table 2: Analytical data of synthesized compounds (IIa ̶ IIm). |
2-Aryl-3- [substituted pyridine-2-yl] methylamino thiazolidin -4-ones
In the synthesis of this series of compounds two steps were involved. Step -I involves the reaction of substituted pyridine hydrazide with different substituted benzaldehydes in equimolar ratio to an intermediate hydrazone (III) which in step-II undergoes cyclocondensation with mercapto acetic acid to form the desired product (IV).
Step-I: Synthesis of N-Methyl-N-[substituted pyridine-2yl] araldehyde hydrazones (III)
The title compound was prepared by refluxing a mixture of 2-[N-methylhydrazino]-3-triflouromethylpyridine (1.91g; 0.01M) and 3-methoxy-4-hydroxy benzaldehyde (1.50g; 0.01M) in methanol for two hours. The reaction mixture was cooled and poured into ice cold water. The resultant compound was filtered, washed, dried and recrystallized from aqueous ethanol.
Yield: 2.31g (71%); M.P.: 151°C
Analysis for C15H14N3O2F3 (Found): C = 55.18; H = 4.53; N = 12.70
(Calculated): C = 55.38; H = 4.31; N = 12.97
IR (KBr)cm-1: 3294 (O-H); 1605 (C=N); 1579, 1519, 1448 (C=C)
Other compounds of this series were synthesized similarly using different substituents R and R‘ as given below:
R R‘
3- CF3 (IIIa) 4-Me; (IIIb) 4-OMe;(IIIc) 4-N(Me)2 ;(IIId)2-NO2
3-CF3, 5-Cl (IIIe) 4-Cl; (IIIf) 4-Me; (IIIg) 4-OMe; (IIIh) 2-NO2;(IIIi) 3-OMe-4-OH
5-CF3,3-Cl (IIIj) ) 4-Cl;(IIIk) 4-Me; (III l) 4-OMe ; (IIIm) 2-NO2; (IIIn) 4-N (CH3)2
The analytical data are presented in Table 3.
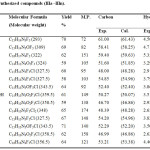 |
Table 3: Analytical studies of synthesized compounds (IIIa ̶ IIIn). |
Sept II: 2-Aryl-3-[substituted pyridin-2-yl] methylamino thiazolidin-4-ones (IV).
A mixture of N-Methyl-N-[3-triflouromethylpyridin-2-yl]-3-methoxy-4-hydroxy benzaldehyde hydrazone (3.25g, 0.01M) and mercaptoacetic acid (0.92g, 0.01M) was refluxed in dioxane (30ml) for 5 hours. Excess of solvent was removed and the residue was poured into ice cold water. This solution was then neutralized from aqueous sodium bicarbonate solution. The compound thus obtained was filtered, washed, dried and recrystallized from aqueous ethanol.
Yield: 2.75g (69%); M.P.: 104°C
Analysis for C17H16N3O3F3 (Found): C = 51.33; H = 4.31; N = 10.30; S=8.20
(Calculated): C = 55.38; H = 4.31; N = 12.90; S=8.02
1R (KBr)cm-1: 3498 (O-H); 1722(C=O); 1579, 1519, 1448 (C=C); 1302 (C-S-C)
Other compounds were prepared similarly using various substituents R and R‘.
R = 3-CF3 R‘ = (IVa) 4-Me;(IVb) 4-OMe;(IVc) 4-N (Me)2;(IVd) 3-NO2
R = 3-CF35-Cl R‘ = (IVe) 4-Cl;(IVf) 4-Me;(IVg) 4-OMe;(IVh) 3-OMe, 4-OH;IV(i) 2-NO2
R = 5-CF3, 3-Cl R‘ =( IVj)4-Cl;(IVk) 4-Me; (IVl) 4-OMe; (IVm) 2-NO2;(IVn) 4-N(Me)2
The analytical data of these compounds are recorded in Table 4.The synthesis of compounds I, II, III and IV is given in Scheme 1.
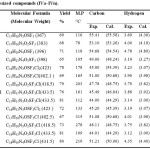 |
Table 4: Analytical studies of synthesized compounds (IVa ̶ IVn). |
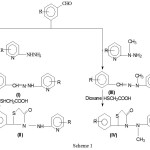 |
Scheme 1 |
Antifungal Screening
All the test chemicals were dissolved in acetone and subjected to the following assay. The standard malt agar medium was prepared by dissolving malt (20g), dextrose (20g) and agar (20g) in 1 litre distilled water and sterilizing at 1.5 kg cm-2 for 20 minutes. After cooling it to about 40°C, a mixture of penicillium G and streptosulphate (5mg each) was thoroughly mixed in order to prevent any bacterial contamination. The filter paper discs (5mm diameter), Whatmann filter paper No. 44 was sterilized in an oven at 120°C for an hour. One mL of the test solution of the toxicant was slowly impregnated on a filter paper disc by evaporating the solvent after each addition with the help of hair drier. The paper disc for control set was similarly impregnated with one ml of solvent. Each disc was finally dried and aseptically transferred to a petri plate (80 mm diameter) containing 10 mL of the culture medium. Only mycelial disc of the test fungus was inoculated upside down in the centre of assay disc. The plates were incubated at 28+1°C for seven days. The experiment was run in triplicate. After 7 days inhibition in fungal growth was determined as a difference in growth between control plate and those treated with test compound. The percentage inhibition of the mycelial growth was calculated by the following equation:
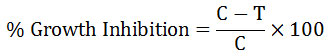
where
C = Diameter of fungus colony (in mm) in control plate
T = Diameter of the fungus colony (in mm) in treated plates.
The percentage of inhibition calculated for selected compounds have been presented in Table 5.
Table 5: % inhibition activity of selected compounds in seven days.
|
Compounds |
Mean %age inhibition |
|||
|
R. Solani |
F. Oxysporun |
|||
|
100 ppm |
10 ppm |
100 ppm |
10 ppm |
|
|
II |
90 |
67 |
93 |
69 |
|
IIa |
70 |
45 |
73 |
44 |
|
IIc |
85 |
62 |
85 |
60 |
|
IIe |
73 |
47 |
61 |
29 |
|
IIi |
74 |
42 |
57 |
22 |
|
IIj |
74 |
44 |
57 |
23 |
|
IIk |
56 |
29 |
53 |
19 |
|
IIl |
51 |
23 |
53 |
20 |
|
IIm |
92 |
75 |
93 |
68 |
| IV |
69 |
40 |
54 |
22 |
|
IVa |
74 |
45 |
70 |
39 |
|
IVb |
84 |
62 |
84 |
61 |
|
IVc |
50 |
23 |
52 |
21 |
|
IVd |
53 |
29 |
51 |
18 |
|
Carbendazim |
96 |
87 |
94 |
86 |
Results and Discussion
Synthesis
All the synthesized compounds were obtained in good yield. The analytical data of the compounds listed in Tables 1-4 are compatible with the composition of desired product. The spectroscopic results are further in support of the compounds. The IR spectra of the compounds demonstrated well resolved absorption bands for characteristic groups. The appearance of absorption bands at 3200cm-1 and at 1620cm-1 in the IR spectra of intermediate hydrazones are clearly assignable to –NH and C=N stretching vibration respectively. The absorption band at 1680-1720cm-1 in the IR spectra of thiazolidinones is due to carbonyl function and the appearance of characteristic bands of C-S-N toxophore at 1250cm-1 are in further conformity of the formation of thiazolidinones.
Antifungal Activity
All the tested compounds showed moderate to high fungicidal activity at 100ppm concentration against the test fungi. The compounds II, IIc, IIm and IVb showed greater than 80% activity against Rhizoctina solani and Fusarium oxysporum. This high activity may be due to the presence of two or more chlorine atoms in the compound and/or due to the methoxy group at para position to the chlorine atom. Although, the compounds bearing hydroxy or carboxylic group also exhibit activity in between 70 to 75% against the fungi but it can be very reasonably concluded that the compounds having combination of –Cl and –OMe at para position exhibit much higher fungicidal activity than any other combination. It was further noticed that neither the replacement of substituent at N- group from –H to –CH3 nor changing the position of –CF3 on pyridine ring from -5 to -3 made any remarkable effect on activity.
Schematic presentation of synthesis of compounds I–IV is given in flow chart (Scheme 1).
References
- Cecchetti, V., Patki, V. M. Eur. J. Med. Chem., 1984, 19, 29.
- Junichi, M., Nishimura, Y. F.R. 2,531,084/1984; Chem. Abst., 1984, 101, 55088.
- Ladner, D. W. U.S.P. 4,459,409/1984 Chem. Abst., 1984, 101, 171123.
- Wopplo, P. J.U.S.P. 4,460, 776/1984 Chem. Abst., 1984, 101, 171115.
- Pieter, T. H., Beatrice, W. S. Eru., Pat. 104, 691/1984 C.A. 1984, 101, 23355.
- Helmut, H., Dieter, K. R. Ger. Officer. Be 3,233,089/1984 C.A. 1984, 101, 23363.
- D’Ascenzio, M., Bizzarri, B., De Monte, C., Carradori, S., Bolasco, A., Secci, D., Rivanera, Faulhaber, N., Bordon, C., Jones-Brando, L. Eur. J. Med. Chem.,2014, 86, 17.
CrossRef - Ulrich, H.,Alexender, K. Eur. Pat. Appl. EP. 431, 421/1991 C.A. 1992, 115, 136120g.
- Singh, S. P., Parma, S. S., Rawan, K. Chem. Rev., 1981, 81, 175.
CrossRef - Singh, S. R. Bokon Bobai 1982, 10, 229.
- Naik, H., Naik, S. K.J. Indian Chem., Sec., 1983, 60, 674.
- Gerjorich, H. J. 63, 287, 467/1966 Chem. Abst., 1967, 66, 28756.
- Goghari, M. H., Parikh, A. R. Indian J. Chem., 1977, 12657, 17.
- Abhinit, M., Ghodke, M., Tratima, N. A. Int. J. Pharm. Pharm. Sci., 2009, 1, 57.
- Yadav, M. R., Murumkar, P. R., Ghuge, R. B. (2008). Vicinal Diary substituted Heterocycles: A gold mine for the discovery of Novel Therapeutic Agents. Elsevier ISBN: 978-0-08-102237-5.
- Kumar, V., Kaur, K., Gupta, G. K., Sharma, A. K. Eur. J. Med. Chem., 2013, 69, 735.
CrossRef - Verma, A., Saraf, S. K. Eur. J. Med. Chem., 2008, 43,897.
CrossRef - Foks, H., Pancechowska-Ksepko, D., Kedzia, A., Zwolska, Z., Janowiec, M., Augustynowicz-kopec, E. Formaco., 2005, 60, 513.
CrossRef - Ren, Q., Mo, W., Gao, L.,He, H., Gu, Y. J. Heterocycl. Chem., 2010, 47, 171.
- Verma, A., Saraf, S. K. Eur. J. Med. Chem., 2008, 43,897.
CrossRef - Foks, H., Pancechowska-Ksepko, D., Kedzia, A., Zwolska, Z., Janowiec, M., Augustynowicz-kopec, E. Formaco., 2005, 60, 513.
CrossRef - El-Wahab, H. A., El-Fattah, M. A., El-Khalik, N. A., Nassar, H. S., Abdelall, M. M. Prog. Org. Coat., 2014, 77, 1506.
CrossRef - Kumar, V., Kaur, K., Gupta, G. K., Sharma, A. K. Eur. J. Med. Chem., 2013, 69, 735.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










