Synthesis Photocatalytic and Antibacterial Activities of Nickle Doped Tio2 Nanoparticles
Ritu Vershney1, Komal Chelaramani2, Arpan Bhardwaj3, Nayma Siddiqui4 and Suresh Kumar Verma5
and Suresh Kumar Verma5
1,2,3Department of Chemistry, Govt. Madhav Science P.G. College, Vikram University, Ujjain (M.P.), India.
4Department of chemistry, Govt. Girls College, Dewas, Vikram University, Ujjain (M.P.), India.
5Department of Chemistry, Govt. Science College, University of Rajasthan, Sikar-332001, India.
Corresponding Author E-mail: arpanbhardwaj11@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340661
Article Received on : 19-09-2018
Article Accepted on : 09-12-2018
Article Published : 12 Dec 2018
The synthesis of Ni doped titania (TiO2) nanoparticles were achieved via simple novel sol gel technique, in which Titanium-n-butoxide and NiCl2 were taken as precursors. Effect of different wt% of dopant in TiO2 was studied on photocatalytic degradation of Aniline blue and Toluidine Blue. The study suggested the increased photocatalytic degradation with increased time duration. The synthesized samples were analyzed by surface electron microscopy (SEM) and X-ray diffraction studies. The antibacterial activity was investigated against Gram-positive Staphylococcus aureus bacteriae. Studies revealed that on increasing the dopant concentration, the diameter of zone of inhibition also increased upto 1.5 wt%.
KEYWORDS:Antibacterial; Nanoparticles; Photocatalytic Activity; Sol-Gel
Download this article as:| Copy the following to cite this article: Vershney R, Chelaramani K, Bhardwaj A, Siddiqui M, Verma S. K. Synthesis Photocatalytic and Antibacterial Activities of Nickle Doped Tio2 Nanoparticles. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Vershney R, Chelaramani K, Bhardwaj A, Siddiqui M, Verma S. K. Synthesis Photocatalytic and Antibacterial Activities of Nickle Doped Tio2 Nanoparticles. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=53826 |
Introduction
Nanoscience and nanotechnology has received great attention for the development of catalyst that exhibits 100% selectivity for the elimination of waste product. This selective process is called as green chemistry. In the modern times the importance of the wastewater treatment, management and its disposal increases gradually and becomes a major health issue. The wastewater treatment can be done by chemical, physical and biological processes.1 Recent studies showed that TiO2, ZnO etc. nanoparticles are used as photocatalysts for the degradation of industrials waste water in the chemical process.2 In a recent investigation of Xiaobo and Samuel, titanium dioxide nanoparticles were used as a photocatalyst for various applications.3 These nanoparticles have great importance in the photodegradation of waste industrial materials and for killing tumour cell in cancer treatment.4 titanium dioxide and zinc oxide nanoparticles have been most widely used for converting toxic harmful organic pollutant to non toxic inorganic molecules like CO2 and water.5 Photocatalytic activities depend on the surface area of nanoparticles. As the surface area of adsorbents increases, the rate of photocatalysis also increases. Chemical modification of surface of nanoparticles overcomes the limitation of nanoparticles as photocatalyst for a particular application. Recent approach for the modification of TiO2 photocatalyst is to dope transition metals into nanoparticles. Dispersion of metal as a dopant in titania nanoparticles generates new energy level in the band gape, resulting increased photoactivity of doped nanoparticles. Doping of metal enhances the trapping of electrons for the inhibition of electron hole pair recombination at the time of photoirradiation. In various investigations different metals have been incorporated into TiO2 nanomaterials.6-9 In this communication we reported the preparation method of titania nanoparticles with different concentration of Ni dopant using sol gel technique and analyzed their effect on photocatalytic decomposition of dyes.
Materials and Method
Titanium n-butoxide (TNBT) (Merck, AR grade), n-Butanaol (CDH AR grade), Acetic acid (CDH, AR grade), Ethanol (Merck, AR grade, 99.9%), glycerol (CDH, AR grade), NiCl2 (CDH, AR grade).
Experimental Procedure
Synthesis of Ni doped TiO2 nanoparticles were done using sol-gel method, adopted by Trung et al.10 Titanium-n-butoxide (TNBT) (5ml) was mixed with equal quantity of n-butanol with continuous stirring on magnetic stirrer to prevent the fast hydrolysis of TNBT. Then NiCl2 solution was added in this solution so as to deposit different wt % of Ni (0.5, 1, 1.5 and 2 wt%) in TiO2 nanoparticles. 36.4 ml Ethanol and 13.6 ml acetic acid as a modifier were taken in RB flask under ice cold condition and the mixture was subjected to stirring for 10 minutes. Then glycerol as gelling agent was added to it. After some time titanium n-butoxide solution was added dropwise in the ice cold solution of ethanol and acetic acid. This mixture was stirred vigorously for three hours at constant temperature of 1-10oC. After three hours of reaction the solution was left at room temperature. Next day the solution was stirred at 600C for 2-3 hours and then kept it at 1000C until the gelling reaction was completed. The powder so obtained was crushed using mortar pestle. The Ni doped TiO2 nanoparticles obtained were calcined in muffle furnace at 400oC.
Results
SEM micrographs were recorded using JEOL, Model JSM 5400 scanning electron microscope. Figure 1 showed the surface morphology of Ni doped TiO2 nanoparticles. The XRD measurements were done using Bruker D8 Advance X-ray diffractometer using Cu Kα, radiation (wavelength=0.154 nm) operated at 40 kV and 40 mA current. The size of nanoparticles and phase composition of the synthesized doped TiO2 samples were carried out using X-ray diffraction analysis. Figure 2 represents the XRD patterns of calcined Ni doped TiO2 powder at 400oC. The photo activities of undoped and Ni doped titania nanoparticles were evaluated by UV-Visible spectrophotometer (Labindia) by degradation of aqueous solution of organic dyes such as Toluidine blue and Aniline Blue.
Discussion
TiO2 nanoparticles have strong tendency to agglomerate so size of doped nanoparticles is not exactly evaluated through SEM. Inhomogeneity is observed in the distribution of nanoparticles.
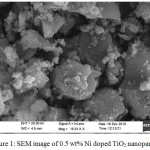 |
Figure 1: SEM image of 0.5 wt% Ni doped TiO2 nanoparticles. |
The peaks of samples at 400oC confirmed that there is an anatase structure at 2θ=25.4o.11 These diffraction patterns do not show any peak regarding rutile phase. The intense and sharp peaks indicate complete crystallization of nanoparticles. No other impurities were observed, which indicated the formation of pure crystalline phase. The average size of the nanoparticles was calculated with the help of Scherrer equation and it was found to be below 200 nm.
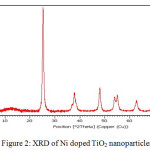 |
Figure 2: XRD of Ni doped TiO2 nanoparticles. |
Photocatalytic Activity
M/1000 stock solutions of dyes were prepared by in 100 ml distilled water. The solution was diluted upto different concentrations. 20 ml of dye solution (selected conc.) was taken and exposed to sun light and during light irradiation the solution was stirred for different time intervals on magnetic stirrer. The reaction mixture collected at different time interval was evaluated by UV visible spectrophotometer to study the photodegradation of dyes. Similar studies were carried out with 0.001gm pure TiO2 and 0.001 gm Ni doped TiO2 nanoparticles. The photocatalytic phenomenon was estimated with the help of UV visible spectrophotometer.
On increasing the dopant concentration the photocatalytic activities increased upto 1 wt% dopant concentration, but sudden fall was observed at higher concentration due to rapid electron hole pair recombination. Figure 3 and 4 showed that on increasing the reaction time the rate of degradation of dyes also increases. Figure 5 and 6 represents the UV-Visible spectra of aniline blue and Toluidine blue in presence of TiO2 catalyst. These spectra clearly revealed that the degradation rate increased on addition of TiO2 nanoparticles. In presence of nanoparticles the absorbance of dyes decreased very rapidly (table 1 and 2). From UV visible spectra (figure 6) it was observed that during photdegradation of toluidine blue in presence of TiO2 nanoparticles the absorbance decreased upto 30 minutes. No considerable change was observed when the reaction mixture was exposed for 40 minutes. Among various transition metal ions Ni2+ was observed to be more efficient dopant for photocatalytic activity of TiO2 nanoparticles.12,13 This high photocatalytic activity of TiO2 nanoparticles is due to enhanced separation of electron hole pair on the surface of TiO2 nanoparticles by Ni2+ dopant. Figure 7 and 8 showed the degradation of dyes with Ni doped TiO2 nanoparticles.
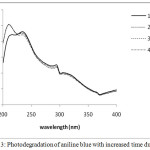 |
Figure 3: Photodegradation of aniline blue with increased time duration. |
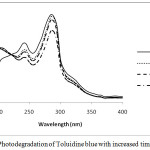 |
Figure 4: Photodegradation of Toluidine blue with increased time duration. |
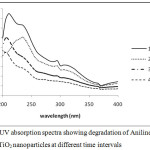 |
Figure 5: UV absorption spectra showing degradation of Aniline blue with undoped TiO2 nanoparticles at different time intervals. |
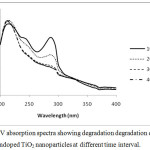 |
Figure 6: UV absorption spectra showing degradation degradation of Toluidine blue with undoped TiO2 nanoparticles at different time interval.Click here to View figure |
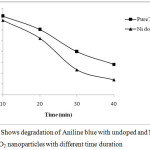 |
Figure 7: Shows degradation of Aniline blue with undoped and Ni doped TiO2 nanoparticles with different time duration. |
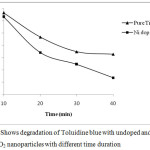 |
Figure 8: Shows degradation of Toluidine blue with undoped and Ni doped TiO2 nanoparticles with different time duration. |
Table 1: Photodegradation of Aniline blue dye in terms of absorbance at 235 nm.
| S.No. | Reaction time | Pure Aniline blue | TiO2 /Aniline blue |
Ni doped TiO2/ Aniline blue |
| 1 | 10 minutes | 0.922 | 0.728 | 0.688 |
| 2 | 20 minutes | 0.907 | 0.604 | 0.522 |
| 3 | 30 minutes | 0.903 | 0.399 | .0.231 |
| 4 | 40 minutes | 0.881 | 0.282 | 0.139 |
Table 2: Photodegradation of Toluidine blue dye in terms of absorbance at 285 nm.
| S.No. | Reaction time | Pure Toluidine blue | TiO2 / Toluidine blue | Ni doped TiO2/ Toluidine blue |
| 1 | 10 minutes | 0.943 | 0.665 | 0.632 |
| 2 | 20 minutes | 0.920 | 0.468 | 0.341 |
| 3 | 30 minutes | 0.885 | 0.348 | 0.245 |
| 4 | 40 minutes | 0.768 | 0.328 | 0.136 |
Antibacterial Activity
Ni doped TiO2 nanoparticles showed good antibacterial action against staphylococcus aureus. The antibacterial activity was performed by well diffusion method. Mueller-Hinton agar (Hi-Media) was prepared, sterilized and poured into petri dish. The medium was allowed for solidification then prepared inoculum was added on the plate aseptically. The inoculum was spreaded by using the sterile L-shaped glass spreader or cotton swab. Well of 6 mm diameter was created with sterilized test tube and then filled with different diluted suspension of materials to be tested (2.5 l). The plates were incubated at 28 for 3 days. The activity is expressed as means of zone of inhibition diameter (mm). Figure 9 showed the effect of different concentration of Ni dopant TiO2 on antibacterial behavior. As the concentration of dopant increased the zone of inhibition increased. But at 2% concentration the diameter of zone of inhibition was found to be decreased due to agglomeration.
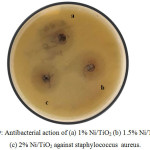 |
Figure 9: Antibacterial action of (a) 1% Ni/TiO2 (b) 1.5% Ni/TiO2 and (c) 2% Ni/TiO2 against staphylococcus aureus. |
Table 3: Zone of inhibition in mm.
|
Bacteria |
Zone of Inhibition (mm) |
|||
| 0.5 wt % Ni/TiO2 | 1 wt % Ni/TiO2 | 1.5 wt % Ni/TiO2 | 2.0 wt % Ni/TiO2 | |
| Staphylococcus aureus | 18 | 20 | 21 | 17 |
Conclusion
Ni/TiO2 nanoparticles were successfully prepared via sol gel technique by taking different wt % of dopant. XRD measurement showed the successful formation of TiO2 nanoparticles. Incorporation of dopant in pure TiO2 increased the photo degradation of dyes and showed good antibacterial activity, so can be used for the degradation of various industrial waste and colored materials.
Acknowledgments
The authors would like to express their gratitude to MPCST, Bhopal for financial support. We are highly thankful to Head of Microbiology Department and their colleagues for providing the facility for performing antibacterial study. Authors are also thankful to the Director and the members of IISER Bhopal, for giving their contribution for the analysis of sample.
References
- Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. J. Hazard. Mater. 2002, 89, 303-317.
CrossRef - Nakata, K.; Fujishimaa, A. J. Photochem. Photobiol. C. 2012, 13, 169-189.
- Chen, X.; Mao, S.S. Chem. Rev. 2007, 107, 2891-2959.
CrossRef - Attia, A.J.; Kadhim, S.H.; Hussen, F.H. J. Chem. 2008, 5, 219-223.
- Akpan, U.G.; Hameed, B.H. J. Hazard. Mater. 2009,170, 520-529.
CrossRef - Frindell, K. L.; Bartl, M. H.; Robinson, M. R.; Bazan, G. C.; Popitsch, A.; Stucky, G. D. J. Solid State Chem. 2003, 172, 81-88.
CrossRef - Bessekhouad, Y.; Robert, D.; Weber, J. V.; Chaoui, N. J. Photochem. Photobiol. A 2004, 167, 49-57.
CrossRef - Bryan, J. D.; Heald, S. M.; Chambers, S. A.; Gamelin, D. R. J. Am. Chem. Soc. 2004, 126, 11640-11647.
CrossRef - Cao, Y.; Yang, W.; Zhang, W.; Liu, G.; Yue, P. New J. Chem. 2004, 28, 218-222.
CrossRef - Trung, T.; Cho, W.J; Ha, C.S. Mater. Lett. 2003, 57, 2746-2750.
CrossRef - Ganesh, I.; Gupta, A.K.; Kumar, P.P.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundarrajan, G. Sci. World J., 2012, 2012, 1-16.
CrossRef - Begum, N.S.; Ahmed, H.M.F.; Gunashekar, K.R. Bull. Mater. Sci. 2008, 31,747-751.
CrossRef - Sreethawong, T.; Suzuki, Y.; Yoshikawa, S. Int. J. Hydrog. Energy, 2005, 30, 1053-1062.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









