In Vitro Cytotoxicity of the Synthesized Gallic Acid Derivatives (N-Alkyl Gallamide) Against Breast MCF-7 Cancer Cells
Maurin Marcelia1, Ade Arsianti2,3 , Jilly Octaria Tagore Chan1, Stevano Julio Wijoyo1, Fadilah Fadilah2,3, Rista Putrianingsih2, Norma Nur Azizah3, Anton Bahtiar4, Hiroki Tanimoto5 and Kiyomi Kakiuchi5
, Jilly Octaria Tagore Chan1, Stevano Julio Wijoyo1, Fadilah Fadilah2,3, Rista Putrianingsih2, Norma Nur Azizah3, Anton Bahtiar4, Hiroki Tanimoto5 and Kiyomi Kakiuchi5
1Faculty of Medicine Universitas Indonesia, Indonesia.
2Department of Medical Chemistry, Faculty of Medicine,Universitas Indonesia, Indonesia.
3Drug Development Research Cluster, Indonesia Medical Education and Research Institute(IMERI), Faculty of Medicine, Universitas Indonesia, Jl. Salemba Raya 6 Jakarta, Indonesia.
4Department of Pharmacology, Faculty of Pharmacy, Universitas Indonesia, Depok, Indonesia.
5Materials Science, Nara Institute of Science and Technology (NAIST), 8916-5 Takayama-cho, Ikoma, Nara, Japan.
Corresponding Author E-mail: arsi_ade2002@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340506
Article Received on : 03-08-2018
Article Accepted on : 04-09-2018
Article Published : 13 Oct 2018
Gallic acid is a phenolic compound distributed in plants and fruits which has been reported to have cytotoxic effect on MCF-7 breast cancer cell line. In this research, we investigated in vitro cytotoxic effect of six synthesized compounds of gallic acid derivatives (N-alkyl gallamide), namely N-methyl gallamide (2); N-ethyl gallamide (3); N-butyl gallamide (4); N-sec-butyl gallamide (5); N-tert-butyl gallamide (6) and N-hexyl gallamide (7) against breast MCF-7 cells by MTT assay. Linear regression analysis is utilized to analyze data to regenerate IC50 value. The results will be compared with gallic acid as an original compound and doxorubicin as a positive control.Among six synthesized compounds, N-tert-butyl gallamide (6) with IC50 value of 2.1 µg/mL, and N-hexyl gallamide (7) with IC50 value of 3.5µg/mL,showed the stronger cytotoxicity against breast MCF-7 cells compared to gallic acid and doxorubicin. Thus,N-tert-butyl gallamide (6) and N-hexyl gallamide (7) are potential to be further developed as a promising anti-breast cancer agents.
KEYWORDS:Breast MCF-7 Cells; Cytotoxicity; Gallic Acid Derivative; MTT Assay; N-Alkyl Gallamide
Download this article as:| Copy the following to cite this article: Marcelia M, Arsianti A, Chan J. O. T, Wijoyo S. J, Fadilah F, Putrianingsih R, Azizah N. N, Bahtiar A, Tanimoto H, Kakiuchi K. In Vitro Cytotoxicity of the Synthesized Gallic Acid Derivatives (N-Alkyl Gallamide) Against Breast MCF-7 Cancer Cells. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Marcelia M, Arsianti A, Chan J. O. T, Wijoyo S. J, Fadilah F, Putrianingsih R, Azizah N. N, Bahtiar A, Tanimoto H, Kakiuchi K. In Vitro Cytotoxicity of the Synthesized Gallic Acid Derivatives (N-Alkyl Gallamide) Against Breast MCF-7 Cancer Cells. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50714 |
Introduction
Breast cancer is a frequent diagnosed malignancy in females, and considerably the second most common human carcinoma in the world. In 2012, there were approximately 1.67 million of breast cancer cases which has increased by more than 20% since the 2008 estimates. It ranks fifth as a cause of death globally with estimated number of 522,000 deaths (13 per 100,000 population) during 2012. Moreover, the death rate of breast cancer in women is higher in less developing areas (324,000 breast cancer deaths in 2012), and lower death rate in more developing areas (198,000 breast cancer deaths in 2012). Indonesia accounted for 17% of female breast cancer deaths within the Asia-Pacific Region in which 116,000 deaths occurred.1 The prognosis for female breast cancer depends on the stage. A five-year relative survival rate reaches until close to 100% for stage 0 and stage 1. This rate moderately declines to 93% and 72% for stage II and stage III consecutively. Furthermore, five-year relative survival rate for female breast cancer patient on stage IV with metastatic is sharply drop to around 22%.2 Treatment choices for brest cancer include Surgery, chemotherapy, and radiotherapy, however these treatments inconstantly successful and could produce unexpected side effects.3 Therefore, in order to improve the survival rate of patient as well as to reduce the side effects of the breast cancer treatment, the search for new molecules which show a stronger therapeutic reaction with minimum side effects compared to established ones are needed. Gallic acid (1) is a phenolic acid also known as 3,4,5-trihydroxybenzoic acid that has water soluble property and various biological activities, including an inhibitory effect against human breast cancer cells.4 Previously, studies have revealed that gallic acid prompted apoptosis in cancer cells as an accompaniment of oxidative stresses from mitochondrial dysfunction, reactive oxygen species (ROS), and an incline in intracellular Ca2+level.5,6 The length of alkyl chain in ester side chain of gallic acid derivatives influenced the hydrophobicity and physicochemical properties of the derivatives. Alkyl ester gallates with longer alkyl chain would become more hydrophobic that will increase its affinity for the lipid bilayer of the cell membrane which allow the alkyl ester derivatives penetrate easier into the cell membrane of cancer cells than gallic acid. As evidence, the ester derivatives presented more favorable pharmacological properties than those for galllic acid itself due to enhancement of carbon atom lenght in alkyl ester side chain. However, a longer carbon chain in alkyl ester moiety causes a greater molecular weight of hydrophobic compound that less dissolved in water.7 Hence, molecular mechanisms underlying the action of gallic acid remain questionable.In the present study, we have done the synthesis of gallic acid derivatives as anti-hepatitis C virus agents.8 Recently, we also accomplished in silico docking study of gallic acid derivatives as inhibitor of malarial dihydrofolate reductase,9 as an inhibitors of hepatitis C virus receptor binding target NS5B,10 as an inhibitor of Bcl-xl anti-apoptotic protein of breast cancer,11 and as an inhibitor of BRAF colon cancer.12 In this work, as to continue research in developing gallic acid derivatives as an anticancer agent, we focused on the cytotoxicity evaluation of N-alkyl gallamide, compound 2-7 (Figure 1) against MCF-7 cells by MTT cell proliferation assay. The synthesis of gallic acid derivatives, N-alkyl gallamide (compound 2-7) applied in this study, have been completed recently by our research team. These alkyl amide derivatives of gallic acid demonstrated a strong cytotoxic effect on colon HCT-116 cells.13
Experimental
Synthesis of Derivative2-7
As reported previously, six gallic acid derivatives, compound 2-7 (Figure 1) were designed by replacing carboxyl group of gallic acid with a series of corresponding alkylamines to generate N-alkyl gallamide 2-7. Four compounds, namely derivative 2, 3, 4 and 7, were designed to have a linear aliphatic carbon chain in N-alkyl benzamide group, whereas compound 5 and compound 6 were designed to have a branched alphatic carbon chain.13
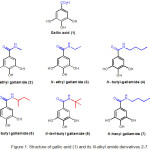 |
Figure 1: Structure of gallic acid (1) and its N-alkyl amide derivatives 2-7. |
Synthesis procedure, experimental data, and synthetic pathway of N-alkyl gallamide 2-7 (Figure 2) have been described in our previous work.13 As summarized in Figure 2, amidation of gallic acid (1) with methylamine, ethylamine, butylamine, sec-butylamine, tert-butyl amine, and hexylamine using a combination of WSCD.HCl/HOBt in the presence of basic NMM afforded the corresponding N-alkyl gallamides, which are N-methylgallamide (2), N-ethyl gallamide (3), N-butyl gallamide (4), N-sec-butyl gallamide (5), N-tert-butyl gallamide (6) and N-hexyl gallamide (7) with a yield ranging from 18% to 84%.13
MTT Assay in Determining the Cytotoxicity
For the MTT assay, 1×10 cells/well were seeded into a 96-well cell plate in a total volume of 100 μL for 24 hours. The medium was then removed, and the cells were treated with the following N-alkyl gallamides: N-methyl, N-ethyl, N-butyl, N-sec-butyl, N-tert-butyl, N-hexyl gallamides at concentrations ranging from 1.5 to 200 μg/mL. After 24-hour incubation, the medium was removed while 100 μL of MTT was added to each well, followed by a 2- to 4-hour incubation period. MTT solution was then discarded and added with 100 μL DMSO. The absorbance was then measured at 630 nm using ELISA reader. Data of cell growth inhibition were provided as the average ± standard deviation, whereas the inhibition rate (Ir) can be calcutalted using formula:
![]()
This procedure was triplicated for each plate. The data obtained will be processed using Microsoft Excel 2016 by extrapolating the concentration of N-alkyl gallamide (in x-axis) versus the percentage of breast cancer cell inhibition (in y-axis), where a linear regression was then created. IC50 value was determined using probit method by plotting the concentration of the sample against percentage of lnhibition.
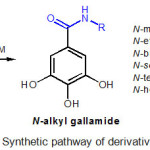 |
Figure 2: Synthetic pathway of derivatives2-7. |
Result and Discussion
In vitro cytotoxicities of 3,4,5-trihydroxy-N-alkyl-benzamides against breast MCF-7 cancer cellsis displayed in Table 1. Cytotoxicities expressed by half maximal inhibitory concentration (IC50) value. The lower IC50 value the stronger cytotoxic activity. Atjanasuppat et al. reported that cytotoxicity of compound were categorized into four groups based on IC50 value. IC50 value below or equal 20 μg/mL, strong cytotoxic activity; IC50 value in range of 20 to 100 μg/mL, moderate cytotoxic activity; IC50 value ranging from 100 to 1000 μg/mL, weak cytotoxic activity; and IC50 value over than 1000 μg/mL, shows no cytotoxic activity.14 From the result of this experiment, as shown in Table 1, all N-alkyl gallamides (compound 2-7) exhibited a strong cytotoxic activity against breast MCF-7 cancer cells as all of the IC50 values were below 20 μg/mL.
Effect of chain length in cytotoxic property
As shown in Table 1, generally the cytotoxicity of derivatives that have a linear aliphatic carbon chain in N-alkyl gallamides greatly improved by addition of carbon chain in N-alkyl moiety. It can be observed from IC50 value of N-methyl gallamide 2 (IC50: 12.6 µg/mL), –N-ethyl gallamide 3 (IC50: 11.3 µg/mL), –N-butyl gallamide 4 (IC50: 10.3 µg/mL) and N-hexyl-gallamide 7 (IC50: 3.5 µg/mL) consecutively, which are decreasing by addition of carbon chain in N-alkyl-benzamide group. This suggested that as the carbon chain is longer, the derivative become more non-polar, its permeability towards cell membrane was greatly increased, which lead to the increasement of its cytotoxicity against MCF-7 cancer cells.
Effect of chain branching in cytotoxic property
Derivatives with a branched carbon chain in N-alkyl benzamide group shows a greater cytotoxicity compared to derivatives with a linear carbon chain. It can be observed on the branched-chain four-carbon of N-sec-butyl gallamide 5 with IC50 value of 8.4 µg/mL, and N–tert-butyl gallamide 6 with IC50 value of 2.1 µg/mL, which have a greater cytotoxicity than N-butyl gallamide 4 (IC50: 10.3 µg/mL) which also has four-carbon chain, but with a linear structure.
Another study that gave similar results was a study conducted by Frey et al., which proved that isobutyl gallate had lower IC50 value compared with butyl gallate against sarcoma 786A,TA3 and TA3-MTX-R tumor cells. Correspondingly, cytotoxic activity of isoamyl and isopropyl gallate were stronger than the cytotoxicity of amyl and propyl gallate.15
Table 1: Cytotoxicities (IC50 in µg/mL) of N-alkyl gallamides 2-7, gallic acid (1), and doxorubicin against breast MCF-7 cells.
| Compound | Cytotoxicity (IC50, µg/mL) |
| Gallic acid (1) | 7.5 |
| N-methyl gallamide (2) | 12.6 |
| N-ethyl gallamide (3) | 11.3 |
| N-butyl gallamide (4) | 10.3 |
| N–sec-butyl gallamide (5) | 8.4 |
| N–tert-butyl gallamide (6) | 2.1 |
| N-hexyl gallamide (7) | 3.5 |
| Doxorubicin | 10.6 |
The addition of branch into the derivative of gallic acid will change the solubility. Chain branching allows the compound to be more lipophilic owing to the decreasing the polarity, thus making it easier to penetrate the lipid bilayer.16
Among the six synthesized derivatives, N-tert-butyl gallamide 6 and N-hexyl gallamide 7 showed a greater cytotoxicity against breast MCF-7 cells than gallic acid (IC50: 7.5 µM) and doxorubicin (IC50: 10.6 µM) as a positive control. These results indicated that modifying the carboxyl group of gallic acid become a branched-alkyl chain with four carbon atom in N–tert-butyl gallamide 6, and become a linear-alkyl chain with six carbon atom in N-hexyl gallamide 7, have successfully improved its cytotoxicity against breast MCF-7 cancer cells. Thus, N–tert-butyl gallamide 6 and N-hexyl gallamide 7 are potential to be further developed as anti-breast cancer drugs.
Conclusion
Six synthesized gallic acid derivatives of N-alkyl gallamide have been evaluated on their basis of cytotoxicity against breast MCF-7 cells. Among six derivatives, N-tert-butyl gallamide (6) and N-hexyl gallamide (7) showed a greater cytotoxicity against breast MCF-7 cells.
Acknowledgements
We sincerely thank to Directorate of Research and Community Engagement (DRPM) and Faculty of Medicine, Universitas Indonesia for the PITTA research grant. We also thank to Division of Materials Science, Nara Institute of Science and Technology (NAIST), Japan, for NAIST Global Collaborative Research Program.
References
- Ferlay, J.;Soerjomataram, I.;Ervik, M.;Dikshit, R.;Eser, S.;Methers, C. GLOBOCAN v1.0, Canceer Incidence and Mortality Worldwide: IARC CancerBase No.11 [Internet]. Lyon, France: International Agency for Research on Cancer, 2013. Available from: http://globocan.iarc.fr.
- American Cancer Society. Breast Cancer Survival Rates by Stage [Internet]. 2014. Available from: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-survival-by-stage.
- Cardoso, F.; Costa, A.; Senkus, E.; Aapro, M.; Andre, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.; Biganzoli, L.; Cardoso, M.J.; Carey, L.3rd ESO-ESMO international consensus guidelines for Advanced Breast Cancer (ABC3). Ann. Oncol.,2017, 28,16-33.
CrossRef - Wang, K.; Zhu, X.; Zhang, K.; Zhu, L.; Zhou, F.J.Biochem. Mol. Toxicol.,2014, 28, 387-393.
CrossRef - Inoue, M.;Sakaguchi, N.;Isuzugwa, K.;Tani, H.;Ogiwara, Y.Biol. Pharm. Bull., 2000,23,1153-1157.
CrossRef - Serrano, A.; Palacios, C.; Roy, G.;Cespon, C.;Villar, M.L;Nocito, M.; Gonzales-Porque, P. Arch. Biochem. Biophys, 1998,.350, 49-54.
CrossRef - Locatelli, C.;Filippin-Monteiro, F.B.;Creczynski-Pasa, T.B.Eur. J. Med. Chem., 2013,60, 233-239.
CrossRef - Arsianti, A.; Aoki-Utsubo,C.; Fadilah, F.; Bahtiar, A.; Apriyanto, D.R.; Dwira, S.; Pradisty, N.A.; Tanimoto, H.; Kakiuchi, K.; Sudarmono, P.; Hotta, H.; Paramita, R.I.; Erlina, L. Asian J. Pharm. Clin. Res.,2017, 10, 164-167.
CrossRef - Arsianti, A.; Astuty, H.; Fadilah, F.; Bahtiar, A.; Tanimoto, H.; Kakiuchi, K. Asian J. Pharm. Clin. Res.,2017, 10, 330-334.
CrossRef - Arsianti, A.; Fadilah, F.; Bahtiar, A.; Dwira, S.; Apriyanto, D.R.; Paramita, R.I Int. J. Chemtech Res.2017, 10, 111-117.
- Paramita, R.I.; Arsianti, A.; Radji, M. Int. J. Chemtech Res., 2017, 10, 348-355.
- Humaedi, A.; Arsianti, A.; Radji, M. Int. J. Chemtech Res., 2017, 10, 310-315.
- Chan, J.O.T.; Arsianti, A.; Marcelia, M.; Wijoyo, S.J.; Fadilah, F.; Putrianingsih, R.; Azizah, N.N.; Tanimoto, H.; Kakiuchi, K.Orient. J. Chem., 2018, 34, 1362-1367.
CrossRef - Atjanasuppat, K.; Wongkham, W.;Meepowpan, P.;Kittakoop, P.;Sobhon, P.; Bartlett, A.; Whitfield. P.J. J. Ethnopharmacol.,2009; 123,475-482.
CrossRef - Frey, C.;Pavani, M.;Cordano, G.; Munoz, S.; Rivera, E.; Medina,J. Comp.Biochem. Phys. A.,2007,146, 520-527.
CrossRef - Mannhold, R.;Kubinyi, H.; Timmerman, H. editors. In: Todeschini, R.;Consonni, V.; Handbook of molecular descriptors methods and principal in medisinal chemistry. 11th ed. German: Wiley-VCH,2008.

This work is licensed under a Creative Commons Attribution 4.0 International License.









