Biopolymer Coated Coreshell Magnetite Nanoparticles for Rifampicin Delivery
Justin Chellapan, Antony Vincent Samrot , An Arun Annamalai, Rai Kamal Bhattacharya, Padmanaban Sathiyamoorthy and Chamarthy Sai Sahithya
, An Arun Annamalai, Rai Kamal Bhattacharya, Padmanaban Sathiyamoorthy and Chamarthy Sai Sahithya
Department of Biotechnology, School of Bio and Chemical Engineering, Sathyabama Institute of Science and Technology, Jeppiaar Nagar, Sholinganallur, Chennai – 600 119, Tamil Nadu, India.
Corresponding Author E-mail: antonysamrot@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340521
Article Received on : 18-07-2018
Article Accepted on : 28-08-2018
Article Published : 10 Oct 2018
In this study, chemically synthesized magnetite was produced where the size was found to be size between 50 and 60nm with para ferro magnetic property. Coreshell magnetite nanoparticles was produced with initial coating of rifampicin by ultrasonication and was encapsulated with any of one among four different biopolymers namely chitosan, starch, casein and polyhydroxybutyrate (PHB). Coreshell nanoparticles were analysed for their drug loading efficiency and drug release studies. PHB and chitosan loaded particles were observed to release drug steadily. All the biopolymer coated nanoparticles were subjected to antibacterial activity against Klebsiella sp by agar well diffusion method. PHB and chitosan coating of the particles were found to be the best for holding rifampicin. Magnetite was subjected for MTT based cytotoxicity assay against peripheral mononuclear cells and its LC50 was found at 60 µg/ml.
KEYWORDS:Chitosan; Drug Delivery; Magnetite; Polyhydroxybutyrate (PHB); Rifampicin
Download this article as:| Copy the following to cite this article: Chellapan J, Samrot A. V, Annamalai A. A, Bhattacharya R. K, Sathiyamoorthy P, Sahithya C. S. Biopolymer Coated Coreshell Magnetite Nanoparticles for Rifampicin Delivery. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Chellapan J, Samrot A. V, Annamalai A. A, Bhattacharya R. K, Sathiyamoorthy P, Sahithya C. S. Biopolymer Coated Coreshell Magnetite Nanoparticles for Rifampicin Delivery. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50469 |
Introduction
Nanotechnology is currently trending and is being extensively researched due to their optical, magnetic, bioimaging,1 electrical properties2 and many more, obtained due to its large surface area to volume ratio. In this broad spectrum of nanotechnology, magnetic nanoparticles, especially iron oxide nanoparticles have attracted a lot interest within the researchers due to its wide range of uses in different fields such as its role as contrasting agents in magnetic resonance imaging (MRI),3 magnetocaloric pumps4, agriculture,5 magnet mediated cell separation and purification,6 magnetically guided drug delivery, magnetocytolysis7, hyperthermia,8,9 environmental remediation,10 stem cell labelling and tracking agents,11,12 chemotherapy13 etc. Iron oxide nanoparticles include a wide range of oxides such as magnetite (Fe3O4), hematite (α-Fe2O3), maghemite (γ-Fe2O3) and other ferrites.14 Among the available iron oxide nanoparticles, magnetite nanoparticles are extensively used due to their super paramagnetic quality and biocompatibility.15 Although MNPs have many uses, the main challenges faced by researches working with magnetite NPs include, stability,16 maintenance of crystallinity of the particles etc. Targeted drug delivery using magnetite makes way for drug delivery at site as well as reduced side effects with controlled release of drug for prolonged time.17 Magnetite nanoparticles coated with polymers have been used to carry doxorubicin and 5-fluorouracil and also evaluated invitro.18-20 For best performance, particles must be moderate sized, spherical, crystalline, biocompatible, superparamagnetic and should render large surface areas for tagging drugs.21 Biopolymer coating of the magnetite nanoparticles provide them stability and also allow sustained and targeted delivery possible with less toxicity and higher biocompatibility. In this study, magnetite nanoparticles were chemically synthesized, coated with drug (rifampicin) and encapsulated with different biopolymers like casein, chitosan, starch and polyhydroxybutyrate (PHB) to hold the drug coated magnetite nanoparticles. The produced coreshell nanoparticles were subjected for different studies such as drug encapsulation efficiency, drug release kinetics and invitro drug release studies against microbes.
Materials and Methods
Chemicals used
Polyhydroxybutyrate (PHB) was purchased from Sigma Aldrich, India. NaOH, FeCl3.6H20 and FeSO4.7H20 were bought from Spectrum chemicals, India. Mueller Hinton agar, HiSep, dialysis tube, RPMI 1640 and MTT were purchased from HiMedia, India, where other chemicals were bought from Qualigens, India.
Chemical Method of Preparation of Magnetite
Synthesis of magnetite nanoparticles was done through chemical co-precipitation method of Mascolo et al22 with some modifications. 2.14 g each of FeSO4.7H20 and FeCl3.6H20 was weighed and dissolved separately in 25 ml nitrogenated MilliQ water. Both the solutions were mixed together and made into precursor iron source solution. 50 ml of 5M NaOH solution (using Milli-Q) was added dropwise to the iron source solution under continuous vigorous manual stirring. The solution was left undisturbed in room temperature for 45 min. The whole solution was filtered using Whatmann no.1 filter and the obtained filtrate was separated using a magnet and washed five times with deionised water in order to bring the pH to a neutral scale. The obtained pellet was dried in hot air oven at 160 0C. Nanoparticles were obtained as powder.
Characterization of Magnetite
Magnetite nanoparticles were subjected for FeSEM-EDX (Carl Zeiss Supra 55, Germany) in order to determine the size. Magnetic property of produced nanoparticles was analysed with Vibrating Sample Magnetometer (Lakeshore, USA, Model 7407). In order to determine the crystalline nature and the form of iron oxide, X-Ray diffraction (Rigaku) was performed.
Coreshell Production
10 ml of distilled water was taken in two test tubes. 0.1% acetic acid in 10ml distilled water was taken in another two test tubes. 10 mg of antibiotic rifampicin was added to all the tubes and sonicated for 30 min at room temperature. 1 mg of starch and casein was added separately to test tubes containing 10 ml distilled water, whereas 1 mg chitosan and polyhydroxybutyrate (PHB) was added into the test tubes containing 0.1% acetic acid (to dissolve chitosan and PHB). 1mg of magnetic nanoparticles was added to all the tubes. All the tubes were again subjected for ultrasonication for an hour at room temperature. Then, the solution was centrifuged and the obtained pellet was lyophilized.
Characterization of Coreshell
The coreshell was subjected to Fourier Transform Infra-Red Spectroscopy (FTIR) Analysis (Shimadzu, Japan) and Scanning electron microscopy analysis (Carl Zeiss Supra 55, Germany).
Drug Encapsulation Efficiency
Immediately after encapsulation of drug by ultrasonication, the entire solution was taken and centrifuged. 0.5ml supernatant was collected and the tube was slowly shaken to disperse the nanoparticles. After every 10th minute, the sample was centrifuged at 5000 g and 0.5 ml sample was collected. The above said procedure was followed till 80th minute. The collected supernatant were read at 460nm (λmax of rifampicin) using UV Spectrophotometer (Systronics).23,24 A graph with time interval in x axis and absorbance in y axis was plotted using the obtained absorbance values.
In Vitro Drug Release Kinetics
Coreshell nanoparticles were subjected for drug release studies by dialysis membrane technique.25 Each biopolymer encapsulated particles (10mg) were taken in dialysis membranes (HiMedia, India). The tied dialysis membranes were introduced to a beaker containing 50ml PBS solution (phosphate buffer solution – pH 6.8). PHB coated nanoparticles containing dialysis bag was immersed into 50 ml acidified PBS (pH 6.4 – made to this pH by adding acetic acid to it). This was done to dissolve PHB in acidic pH and to release the drug. 1ml of sample was collected from the beaker at every 15th min time interval for 3h and subjected for absorbance at 460nm in UV visible Spectrophotometer (Systronics). A graph with time interval in x axis and absorbance in y axis was plotted.
Anti-Bacterial Activity
In Vitro drug release studies and bactericidal activity for coreshell nanoparticles was done against Klebsiella sp by agar well diffusion method.26 Mueller Hinton agar plate was swabbed all over with Klebsiella sp. Wells were made using gel punching kit. Particles were dispersed in PBS and different concentrations of particles (10µg, 20µg, 30µg and 40µg) were poured onto the wells. After 48h, the zone of clearance was measured and recorded.
MTT Based Cytotoxicity Assay
Isolation of Peripheral Blood Mononuclear Cells (PBMC)27
2ml of intravenous blood sample was collected and transferred to a tube containing EDTA. Blood was added with 2ml of RPMI 1640 media (without serum). It was carefully overlaid on lymphocyte separation solution (HiSep) (HiMedia, India) and centrifuged at 8000 rpm for 10 minutes. The interphase of the content was collected and added to RPMI 1640 (without serum) and centrifuged again at 8000rpm for 10 min. To the obtained pellet, 1 ml of RPMI 1640 (with 10% serum) was added and this consisted of PBMC.
MTT assay28
The PBMC suspension was diluted with RPMI 1640 (with 10% serum) (HiMedia, India) in order to get 1 X 10³ cells/ 20µl suspension. These cells were then added to 96 well tissue culture plate. The content was made upto 100 µl using RPMI 1640 (with 10% serum). The 96 well plate was covered and incubated in a humidified atmosphere at 37 ºC for 3 h in 5% CO2 incubator to make the cells to adapt to the condition. After the incubation time, the nanoparticles were dispersed in RPMI 1640 medium (with 10% serum) and were treated with different concentrations of core-shell nanoparticles (i.e., 20, 40, 60 and 80µg). Every well was made upto 200 µl using fresh medium and was incubated for 24h. After incubation, 15 µl MTT (5mg/ml) was added to all wells and were incubated for 4h in CO2 incubator at 37ºC. The contents of the well were aspirated and 200 µl of DMSO was added. The absorbance was recorded at 570 nm. The graph was plotted with percentage cytotoxicity (Y-axis) against the concentration of drug (X-axis).
Percentage cytotoxicity was calculated by the following formula,

Result and Discussion
Characterization of Magnetite
The size of the nanoparticles as per the Fe-SEM analysis was found to be between 50 nm and 60 nm while, the chemical composition of the synthesized particles were found to have Fe3+ and O4 in it using EDX analysis (Figure 1). Nanoparticles of size below 100 nm are suitable for drug delivery or drug carrier,29-31 thus the produced magnetite can be used to form core shell nanoparticles for drug delivery.
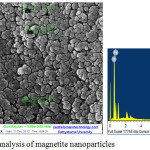 |
Figure 1: FeSEM-EDX analysis of magnetite nanoparticles. |
Magnetite nanoparticles were found to possess para-ferromagnetic activity by VSM (Vibrating Sample Magnetometer) analysis (Figure 2). The saturation magnetization of Fe3O4 particles at 2500 G were found to be 2.4 emu/g, which are in good agreement with magnetic data reported on magnetite. Varma et al32 reported the saturation magnetization of chemically synthesised Fe3O4 particles at 8000 G was found to be 2.4 emu/g. Very lower i.e. ~50 emu/g was reported in MC-IOPs by Bhattarai et al (2008).33
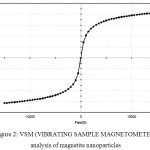 |
Figure 2: VSM (VIBRATING SAMPLE MAGNETOMETER) analysis of magnetite nanoparticles. |
The peaks formed found by XRD analysis were indexed to (220), (311), (400), (511) indicating the characteristic graph of cubic crystalline structure of magnetite nanoparticles (Fe3O4) (Figure 3), which is on par with the earlier reports.22,34,35
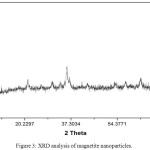 |
Figure 3: XRD analysis of magnetite nanoparticles. |
Characterization of Coreshell Nanoparticles
FTIR spectra of magnetite nanoparticles showed an IR band at 3356 cm-1 and 2900 cm-1 which is for O- stretching mode. IR band at 1629 cm-1 was due to H2O bending vibrations. The FT-IR spectrum of substoichiometric magnetite Fe3O4 showed two IR bands at 507 and 474 cm-1. Thus, the produced magnetite has found to be having the chemical formula of Fe3O4 (Figure 4a). IR bands at 565 and 360 cm-1 were reported for vibration modes in Fe3O4 36-38. Absorption bands of chitosan was observed at 3360 cm-1 which depicted the O–H and N–H stretching vibrations. The other peaks such as ∼2800 cm-1 (C–H), 1653 cm-1 (N–H), and 1080 cm−1 (C–O–C) were also the characteristics of chitosan (Figure 4b).39-41 IR spectra of PHB showed the bands at 1100 and 1273 cm-1 representing C-O-C while band at 1380 cm-1 represents -CH3 and the band at 1641 cm-1 for C=O stretching of ester carbonyl group (Figure 4c).42 In casein encapsulated particles 1544-1527 cm-1, for N-H bending vibrations of amide and 1641 cm-1 for C=O stretch of peptide bond (Figure 4d).43 Bands between 850 and 500 cm-1 for C-C stretching and C-H bending were for anhydroglucose unit of starch. The peak near 1000 cm-1 corresponds to -C-O-C- bonds while the peaks at 2650 cm-1 and ∼3300 cm-1 were due to the characteristic vibrations of C-H and -OH bonds (Figure 4e).44
 |
Figure 4: FTIR analysis of magnetite nanoparticles coated with antibiotic and biopolymers. |
a) FTIR analysis of magnetite b) magnetite + rifampicin + coated with chitosan, c), magnetite + rifampicin + coated with polyhydroxy butyrate, d) magnetite + rifampicin + coated with casein e) magnetite + rifampicin + coated with starch.
When the magnetite was subjected for coreshell formation i.e coating with antibacterial agent (rifampicin) and biopolymer, the size of the particle was found to increase as studied through SEM analysis, where it was higher with chitosan coating (Figure 5a). Amongst all the biopolymer used, polyhydroxyalkonate coated nanoparticles were found to be smaller in size (80-90nm) (Figure 5b).
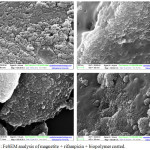 |
Figure 5: FeSEM analysis of magnetite + rifampicin + biopolymer coated. |
a) magnetite + rifampicin + coated with chitosan, b), magnetite + rifampicin + coated with polyhydroxy butyrate, c) magnetite + rifampicin + coated with casein d) magnetite + rifampicin + coated with starch.
Drug Encapsulation Efficiency
All the biopolymers could encapsulate the drug in less than 30 minutes (Figure 6). It might be because the drug and biopolymer coating were subjected for 1h of sonication, whereas Márquez et al found that SiO2-magnetite microspheres took 9 h of contact time when being coated with 0.413 mg Rhodamine B and 0.343 mg methotrexate at 40°C.45
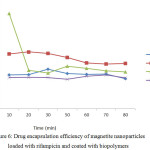 |
Figure 6: Drug encapsulation efficiency of magnetite nanoparticles loaded with rifampicin and coated with biopolymers. |
In vitro Drug Release Kinetics
PHB and chitosan were found to release drug slowly and steadily for 3 h (Figure 7), other polymers such as casein and starch was releasing faster since they got solubilized in water. Hence, PHB and Chitosan biopolymers were found to be good encapsulating agents as they release slowly and efficiently. Samrot et al46 also reported chitosan based microparticles to release curcumin for 3 h. Magnetic-chitosan nanogels have also been reported to release doxorubicin over 72 h.47
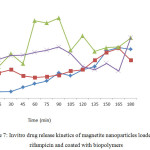 |
Figure 7: Invitro drug release kinetics of magnetite nanoparticles loaded with rifampicin and coated with biopolymers. Click here to View figure |
Antimicrobial Activity
Among all the biopolymers used in this study, chitosan and PHA were found to be the best for holding the antibacterial compound i.e. rifampicin (Table 1). Chitosan coated particles were not showing antibacterial activity since the organism lacks enzymes to degrade chitosan, but PHA might have been utilized by Klebsiella sp, as it might possess enzyme like depolymerases to degrade PHA.48 Casein and starch coated particles were showing uneven results, which might be due to solubilizing nature in water.
Table 1: Antibacterial activity of core shell – Magnetite nanoparticles coated with antibiotic and biopolymer against Klebsiella.
| Concentration(µg/µL) | Zone of Inhibition(cm) | |||
| rifampicin+ coated with chitosan | rifampicin + coated with polyhydroxy butyrate | rifampicin + coated with casein | rifampicin + coated with starch | |
| Positive control | 0.7 | 0.8 | – | – |
| Negative control | – | – | – | – |
| 10 | 0.1 | 1.3 | 2.0 | 1.0 |
| 20 | 0.1 | 1.6 | 2.0 | 1.3 |
| 30 | 0.1 | 1.9 | 2.2 | 1.4 |
| 40 | 0.2 | 2.5 | – | 1.5 |
Cytotoxicity Assay
Magnetite nanoparticles were found to have LC50 at 60µg/ml concentration against the normal blood cells i.e. peripheral blood mononuclear cells. Thus, the concentration below 60µg/ml could be used for the drug delivering study invitro (Figure 8). It has been noticed that magnetic nanoparticles are not affecting cell viability, proliferation, or differentiation capacity of stem cells and few studies were only there to report adverse or toxic effects of magnetite nanoparticles.49,50
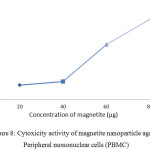 |
Figure 8: Cytoxicity activity of magnetite nanoparticle against Peripheral mononuclear cells (PBMC). Click here to View figure |
Conclusion
Magnetite was produced by chemical co-precipitation method and the size of the nanoparticle was found to be between 50 and 60nm. The coreshell nanoparticle was produced by coating magnetite nanoparticles with antibiotic – rifampicin and encapsulated with biopolymers like chitosan, PHB, starch and casein. Both the chitosan and PHB coated particles were found to show a slow and steady release of drug. Antibacterial activity of the coreshell particles was assessed using Klebsiella sp where, PHB showed a stable zone of inhibition around the well whereas, chitosan did not release any drug as there was no zone of inhibition. LC50 value of the produced nanoparticles was found to be 60µg/ml against PBMC cells.
Funding
There is no funding source
Conflict of Interest
There is no conflict of interest
Acknowledgement
The authors acknowledge the Management, Sathyabama Institute of Science and Technology
References
- Gayathri, A., Mitra, A.K., Cholkar, K. Nanosystems for Diagnostic Imaging, Biodetectors, and Biosensors. In: Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices. 2017.
- Khan, I., Saeed, K., & Khan, I. Arabian Journal of Chemistry. 2017. https://doi.org/10.1016/j.arabjc.2017.05.011.
- Schütt, D. Magnetite colloids for drug delivery and magnetic resonance imaging. Institute Angewandte Polymer forschung: Thesis. 2004
- Love, L.J., Jansen, J.F., McKnight, T.E., Roh, Y., Phelps, T.J. IEEE Transactions on NanoBioscience. 2004, 3(2), 101 – 110.
CrossRef - Mukhopadhyay, S.M. Nanotechnology, Science and Applications. 2014, 7, 63–71.
CrossRef - Honda, H., Kawabe, A., Shinkai, M., Kobayashi, T. Journal of Fermentation and Bioengineering. 1998, 86, 191-196.
CrossRef - Roger, J., Pons, J.N., Massart, R., Halbreich, A., Bacri, J.C. Eur. Phys. J. AP. 1999, 5, 321-325.
CrossRef - Rodzinski, A., Guduru, R., Liang, P., Hadjikhani, A., Stewart, T., Stimphil, E., Runowicz, C., Cote, R., Altman, N., Datar, R., Khizroev,S. Scientific Reports. 2016, 6, 20867.
CrossRef - Moroz, P., Jones, S.K., Gray,B.N. Int. J. Hyperthermia. 2002, 18(4), 267-84.
CrossRef - Elliott, D.W., Zhang,W.X. Environ. Sci. Technol. 2001, 1535(24),4922-6.
CrossRef - Mahmoudi, M., Hosseinkhani, H., Hosseinkhani, M., Boutry, S., Simchi, A., Journeay, W.S., Subramani, K., Laurent, S. Chemical Reviews. 2010, 111(2), 253-280.
CrossRef - Horák, D., Babic, M., Jendelová, P., Herynek, V., Trchová, M., Pientka, Z., Pollert, E., Hájek, M., Syková, E. Bioconjugate chemistry. 2007, 18(3), 635-644.
CrossRef - Mahmoudi, M., Sant, S., Wang, B., Laurent, S., Sen, T. Advanced drug delivery reviews. 2011, 63(1-2), 24-46.
CrossRef - Cornell, R. M., Schwertmann, U. The iron oxides: structure, properties, reactions, occurrences and uses. John Wiley & Sons (2003).
CrossRef - Majewski, P., Thierry, B. Critical Reviews in Solid State and Materials Sciences. 2007, 32(3-4), 203-215.
CrossRef - Kalska-Szostko, B., Wykowska, U., Satuła, D., Zambrzycka, E. Colloids and Surfaces B: Biointerfaces. 2014, 113, 295-301.
CrossRef - Belikov, V.G., Kuregyan, A.G., Ismailova, G.K. Pharmaceutical Chemistry Journal. 2002, 36(6), 333–336.
CrossRef - Chang, Y., Bai, Y., Teng, B., Li, Z. Chinese Science Bulletin. 2009, 54(7), 1190–1196.
- Chandra, V., Park, J., Chun, Y., Lee, J.W., Hwang, I., Kim, K.S. ACS Nano. 2010, 4 (7), 3979–3986.
CrossRef - Li, X., Li, H., Liu, G., Deng, Z., Wu, S., Li, P., Xu, Z., Xu, H., Chu, P.K. Biomaterials. 2012, 33, 3013-3024.
CrossRef - Guo, S., Li, D., Zhang, L., Li, J., Wang, E. Biomaterials. 2009, 30(10), 1881–1889.
CrossRef - Mascolo, M.C., Pei, Y., Ring, T.A. Materials. 2013, 6, 5549-5567. doi:10.3390/ma6125549.
CrossRef - Parize, A.L., Stulzer, H.K., Laranjeira, M.C.M., Brighente, M.D.C., de-Souza,T.C.R. Quı´m Nova. 2012, 35(6),1127–1132.
CrossRef - Benetton, S.A., Kedor-Hackmann, E.R.M., Santoro, M.I.R.M., Borges, V.M. Talanta. 1998, 47(3), 639–643.
CrossRef - Hua, S. Int J Nanomed. 2014, 9, 735–744.
CrossRef - Buzia, O.D., Dima, C., Dima, S. Farmacia. 2015, 63(6).
- Hudson,l,, Hay, FC. The lymphocyte: its role and function. In: Practical Immunology. Blackwell Scientific Publications. 1989, 3, 86-94.
- Mosmann, T. J. Immunol. Methods. 1983, 65, 55-63.
CrossRef - Cascone, M.G., LazzeriL,.Carmignani, C., et al. J. Mater. Sci. Mater. Med. 2002, 13, 523–526.
CrossRef - Baran, E.T., Özer, N., Hasirci, V. J. Mater. Sci. Mater. Med. 2002, 13, 1113–1121.
CrossRef - Jong, W.H.D., Borm, P.J.A. Int J Nanomedicine. 2008, 3(2), 133–149.
CrossRef - Varma, M.V.K., Amareshwar, P., Devara, R.K. Inter.J.Drug Delivery. 2011, 3, 101-108.
CrossRef - Bhattarai, S.R., Kc, R.B., Kim, S.Y., Sharma, M., Khil, M.S., Hwang, P.H., Chung, G.H., Kim, H.Y. J. Nanobiotechnol. 2008, 6(1), 1.
CrossRef - Gnanaprakash, G., Philip, J., Jayakumar, T., Raj, B. J. Phys. Chem. 2007, 111, 7978–7986.
CrossRef - Aliramajia, S., Zamaniana, A., Sohrabijama, Z. Procedia Materials Science. 2015, 11, 265 – 269.
- Ishii, M., Nakahira, M., Yamanaka, T. Solid State Communications. 1972, 11(1), 209-212.
CrossRef - Ellid, M.S., Murayed, Y.S., Zoto, M.S., Music, S., Popović, S. Radioanal. Nucl. Chem. 2003, 258, 299.
CrossRef - Gotic, M., Music, S. Journal of Molecular Structure. 2007, 834–836, 445–453.
CrossRef - Gregorio-Jauregui, K.M., Pineda, G.M., Rivera-Salinas, J.E., Hurtado, G., Saade, H., Martinez, J.L., Ilyina, A., Lopez, R.G. Journal of Nanomaterials. 2013, 2012, 813958.
- Guo, L., Liu, G., Hong, R.Y., Li, H.Z. Marine Drugs. 2010, 8(7), 2212–2222.
CrossRef - Chen, L., Tang, C.Y., Ning, N.Y., Wang, C.Y., Fu, Q., Zhang, Q. Chinese Journal of Polymer Science. 2009, 27 (5), 739–746.
CrossRef - Torres, M.G., Paneque, M.R., Zaldivar, M.P. Nucleus. 2008, 39, (25).
- Maddinedi, S.B., Mandal, B.K., Vankayala, R., Kalluru, P., Tammina, S.K., Kumar, H.A.K. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2014, 126, 227–231.
CrossRef - Ochoa, N., Bello, M., Sancristóbal, J., Balsamo, V., Albornoz, A., Brito, J.L. Mat. Res. 2013, 16(6).
CrossRef - Márquez, F., Herrera, G.M., Campo, T., Cotto, M., Ducongé, J., Sanz, J.M., Elizalde, E., Perales, O., Morant, C.:Preparation of hollow magnetite microspheres and their applications as drugs carriers. Nanoscale Res. Lett. 2012, 7(1), 210.
CrossRef - Samrot, A.V., Akanksha, Jahnavi, T., Padmanaban, S., Philip, S.A., Burman, U., Rabel, A.M. Appl. Nanosci. 2016, 6(8), 1219-1231.
CrossRef - Sadighian, S., Hosseini-Monfared, H., Rostamizadeh, K., Hamidi, M. Adv. Pharm. Bull. 2015, 5(1), 115–120.
- Apparao, U., Krishnaswamy, V.G. International Journal of Environmental Bioremediation & Biodegradation. 2015, 3(2), 54-61.
- Mahmoudi, M., Simchi, A., Imani, M.,Shokrgozar, M.A., Milani, A.S., Häfeli, U.O., Stroeve, P. Colloids and Surfaces B. 2010, 75(1).
CrossRef - Markides, H., Rotherham, M., Haj, A.J.E. Journal of Nanomaterials. 2012, 614094.

This work is licensed under a Creative Commons Attribution 4.0 International License.









