Preparation and Conductivity of La9.33 Si6O26 (LSO) - Ce0.85Gd0.15O1.925 (CGO15) Composite Based Electrolyte for IT-SOFC
Atiek Rostika Noviyanti1 , Iwan Hastiawan1, Diana Rakhmawaty Eddy1, Muhammad Berlian Adham1, Arie Hardian2 and Dani Gustaman Syarif3
, Iwan Hastiawan1, Diana Rakhmawaty Eddy1, Muhammad Berlian Adham1, Arie Hardian2 and Dani Gustaman Syarif3
1Department of Chemistry, Faculty of Mathematic and Natural Sciences, Universitas Padjadjaran, Indonesia.
2Department of Chemistry, Faculty of Mathematic and Natural Sciences, Universitas Jenderal Achmad Yani, Jl. Terusan Jenderal Sudirman, Cimahi, Indonesia.
3PSTNT – BATAN, Jl. Taman Sari 71 Bandung 40132 Indonesia.
Corresponding Author E-mail: atiek.noviyanti@unpad.ac.id
DOI : http://dx.doi.org/10.13005/ojc/3404053
Article Received on : 14-07-2018
Article Accepted on : 02-08-2018
Article Published : 27 Aug 2018
Reducing a high-operating temperature of solid oxide fuel cell (SOFC) to intermediate temperature SOFC (IT-SOFC, 500-750ºC) poses a great challenge in the sense of developing solid electrolyte at intermediate temperature range. In response to this, we report a novel composite La9.33Si6O26 (LSO) - Ce0.85Gd0.15O1.925 (CGO) in this study. The synthesis of LSO-CGO composite was carried out by combining LSO with CGO (9:1, 8:2, and 7:3 in weight ratio) using solid state reaction method. In order to get a dense pellet, all of the products were sintered at 1500°C for 3 h. The X-ray diffraction pattern of sintered pellets show typical patterns for both of LSO and CGO which indicate that the composite was successfully formed. The highest conductivity was detected in 7LSO-3CGO, i.e. 2.10×10-3 S cm-1 at 700 ○C and also has low activation energy (0.60 eV). This result suggests that the LSO-YSZ composites are good oxide ion conductors and may potentially be used as an alternative solid electrolyte in IT-SOFC technology.
KEYWORDS:Apatite; Composites; Electrolyte; LSO-CGO, IT-SOFC
Download this article as:| Copy the following to cite this article: Noviyanti A. R, Hastiawan I, Eddy D. R, Adham M. B, Hardian A, Syarif D. G. Preparation and Conductivity of La9.33 Si6O26 (LSO) - Ce0.85Gd0.15O1.925 (CGO15) Composite Based Electrolyte for IT-SOFC. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Noviyanti A. R, Hastiawan I, Eddy D. R, Adham M. B, Hardian A, Syarif D. G. Preparation and Conductivity of La9.33 Si6O26 (LSO) - Ce0.85Gd0.15O1.925 (CGO15) Composite Based Electrolyte for IT-SOFC. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48829 |
Introduction
One of the causes of energy crisis is due to the high usage of fossil fuel as energy source for various human activities. Fuel cell is an alternative technology not to only produce electrical energy, but for its environmentally friendly and efficient property. Fuel cell is an electrochemical device that converts chemical energy from fuel (usually hydrogen) and oxidant (usually air) into electrical energy.1-3
Many types of fuel cells had been increasingly developed in many countries such as USA, Germany, England, and also developing Asian countries such as Japan, Korea, and China. Up to now, there are six types of fuel cells that depend on electrolyte material, which are Alkaline Fuel Cell (AFC), Polymer Electrolyte Membrane Fuel Cell (PEMFC), Phosphoric Acid Fuel Cell (PAFC), Direct Methanol Fuel Cell (DMFC), Molten Carbonate Fuel Cell (MCFC), and Solid Oxide Fuel Cell (SOFC).1,2 Solid Oxide Fuel Cell (SOFC) has attracted many researchers’ attention due to its advantages compared to the others. This includes its capability of using many types of fuel not only H2 but also hydrocarbon, while proving to be low in its maintenance cost and presents high efficiency (could reach 85% when combined with gas turbine).1,2 SOFC also uses high operational temperature (800-1000°C) due to the type of its electrolyte. This high operational temperature causes problem such as unexpected reactions between the materials which lead to lower efficiency. Therefore, the development of SOFC technology by lowering the operational temperature to the intermediate range which is 600-700 °C is seen as a strategic move. Lowering the operational temperature of SOFC has many advantages including the usage of low cost material such as metal as interconnect material, the lowering of fabrication cost and maintenance cost, and avoiding the unexpected reactions at high temperatures that lead to short SOFC lifetime.3
Electrolyte material is the key in order to determine the operational temperature of SOFC. Apatite-based electrolyte has a great potential to be used as electrolyte at Intermediate Temperature SOFC (IT-SOFC) due to its high conductivity. Apatite has the general chemical formula M10(SiO4)6O2±y, in which M is usually derived from rare earth metal group or alkaline earth. Lanthanum is one of elements in rare earth metal groups which has large atomic radius and has a potential in obtaining good conductivity in apatite. Apatite with La called lanthanum silicate-based apatite becomes one of the apatite-based research that attracts many researchers’ attention.4
In our previous works, La9.33Si6O26 have been successfully synthesized via hydrothermal method using La2O3 and Na2SiO3 as the precursors and NaOH as the base which is heated at 240 °C for 3 days.5 The highest conductivity of the obtained apatites was 2.95 x 10-4 S cm-1 at 500°C. However, that value is still low to be used as IT-SOFC electrolyte. Chao-feng et al. reported that the mechanical properties of apatites were unstable, brittle, and hygroscopic.6 These weaknesses made the apatite-based electrolytes to be unsuitable for IT-SOFC electrolyte.
Yttria Stabilized Zirconia (YSZ) is the common SOFC electrolyte and is already commercially available. YSZ worked optimally at high-operating temperatures at 800-1000 °C. YSZ has also good mechanical properties that could support the SOFC cell and has suitable thermal expansion coefficient (TEC) with the other SOFC components especially electrode. However, YSZ could react with nickel-based anode at high operational temperature that effects the efficiency of the cell.
In general, there are two ways to improve the quality of electrolyte so that it can be used as IT-SOFC electrolyte. The first way is finding the other type of metal oxide which has high conductivity within the IT range. The first way of generating process is by modifying the microstructure of metal oxide. The second way is the use of composite material which is an easier way compared to the first one.6
In our previous work, composite apatite and YSZ had been successfully synthesized.7 The conductivity of composite increased when the weight percentage of YSZ increased. The highest conductivity of composite was reached at a composition of 70% LSO (lanthanum silicatebased apatite) and 30% YSZ which is 1.72×10-4 S cm-1 at 500°C.
Another oxide which has a great potential to be used as IT-SOFC electrolyte is ceria-based electrolyte. Doped cerium oxide (Ceria) has higher conductivity at intermediate temperature range compared to the commercial SOFC electrolyte, i.e. Yttria Stabilized Zirconia (YSZ). Doped ceria also has good mechanical properties and suitable thermal expansion coefficient (TEC) with the other SOFC component especially electrode. In our previous research, we worked with ceria-based electrolyte, whereby Gadolinium Doped Ceria (CGO) was successfully synthesized via sol gel method using nitrate salt as the precursor and citric acid monohydrate as the chelating agent.8 The conductivity of the obtained CGO15 (Ce0.85Gd0.15O1.925) was 4.57×10-2 S cm-1 at 700°C, which is suitable to be used as IT-SOFC electrolyte. Ceria-based electrolyte also has good mechanical properties.
In other work, the use of doped ceria in composite strategy could increase the conductivity and the mechanical properties simultaneously of the obtained composite.6 Therefore, the composite strategy is good in order to create new electrolyte material which has high conductivity and good mechanical properties.
In this work, we report the synthesis and the electrical properties of CGO-LSO composited based electrolyte. The effect of weight percent through the crystal structure, morphology, density, and conductivity of CGO-LSO composite based electrolyte were also discussed.
Experiment
To prepare the composite materials, three batches with the LSO to CGO (Ce0.85Gd0.15O1.925) weight ratios of 9:1, 8: 2, and 7: 3 were designed (recorded as 9LSO-1CGO, 8LSO-2CGO, and 7LSO-3CGO respectively). CGO was synthesized by sol-gel using starting material of CeO2 99.99% (trace metal basis. Aldrich) and Gd2O3 99.99% (trace metal basis. Aldrich). LSO was synthesized by hydrothermal using high-purity La2O3 (Aldrich, 99.999%), Na2SiO3 (Sigma, 97%), and a 3 M NaOH solution was used in the preparation of these apatitesas mineralizer. According to the weight ratios, LSO powder and CGO powder were weighted and mixed using ball mill for 60 minutes. Three batches of mixture powders were pressed under 6000 kg m-2 to obtain green pellets with 8 mm diameter and ~2 mm thickness. The pellets were then sintered at 1500 ºC for 3 h. The densities of the sintered pellets were calculated using the ratio of weight and volume (based on their dimension).
The structure identification was examined by using X-ray diffraction (Rigaku SmartLab X-Ray Diffractometer using Cu-Kα radiation (λ = 1.5406 Å)) at room temperature, while the morphology of the calcined powders and sintered pellets were investigated by using scanning electron microscopy (Hitachi-EDAX Team). Meanwhile, the density of the apatite was examined by using the Archimedes principle. Ionic conductivity was measured using an LCR meter (GW Instek 61056) at 600 – 700 ºC and a frequency range of 20 Hz – 5 MHz on the sintered pellet sample. The total resistance (R) after which the value of resistivity (ρ) is calculated by the following equation:
![]()
The surface area (A) and thickness (l) were obtained by measuring the diameter (d) and thickness (l) pellets directly. After the value of resistivity (ρ) was obtained. Finally, the value of conductivity (σ) is calculated using the following equation:
![]()
Results and Discussions
Figure 1 shows the XRD patterns of the as-calcined composite of LSO-CGO. Significant peaks for LSO appeared at 2θ = 21-22 º; 31o; and 45o, while for CGO at 2 θ = 28.5 º; 33o; 47.4o and 56o. The diffraction patterns of the three sintered pellets (1.500 ºC for 3 hours) of 9LSO-1CGO, 8LS0-2CGO, and 7LSO-3CGO exhibit typical LSO and CGO phases. The X-ray diffraction results indicate that all the composites have been successfully prepared.
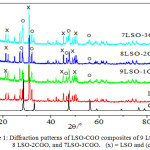 |
Figure 1: Diffraction patterns of LSO-CGO composites of 9 LSO-CGO, 8 LSO-2CGO, and 7LSO-3CGO. (x) = LSO and (o) = CGO.
|
In order to evaluate the relation between the microstructure of composite and conductivity, SEM measurements were carried out. The SEM micrographs of the sintered LSO-CGO are presented in Figure 2. The size of grains was in the range of 3 to 7 μ m. It is not clear whether the LSO and CGO phase is located at the grain boundaries or inside of grains of the main phase. The addition of CGO on LSO reduced the reactivity of sintering powder with the atmospheric H2O and CO2.9 Table 1 listed the EDS data for LSO-CGO composite composition. In overall, the experimental ratio of constituent atoms in (La9.33Si6O26-Ce0.85Gd0.15O1.925) is close to the calculated ratio. There were no impurities detected and it was concluded that the LSO-CGO composite has been well-formed.
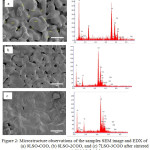 |
Figure 2: Microstructure observations of the samples SEM image and EDX of (a) 9LSO-CGO, (b) 8LSO-2CGO, and (c) 7LSO-3CGO after sintered at 1.500°C for 3 h. |
Table 1: EDS data for LSO-CGO composite composition.
|
Element |
(9LSO-1CGO) |
(8LSO-2CGO) |
(7LSO-3CGO) |
|||
|
Calculation Mass/ % |
EDS/ % |
Calculation Mass/ % |
EDS/ % |
Calculation Mass/ % |
EDS/ % |
|
|
Lanthanum |
62.20 |
62.48 |
55.36 |
56.61 |
48.47 |
49.75 |
|
Silicon |
8.09 |
7.30 |
7.20 |
7.63 |
6.30 |
7.15 |
|
Oxygen |
21.80 |
21.76 |
21.38 |
20.58 |
20.95 |
21.29 |
|
Cerium |
7.11 |
6.67 |
13.98 |
12.07 |
20.86 |
18.38 |
|
Gadolinium |
1.41 |
1.79 |
2.77 |
3.11 |
4.13 |
3.43 |
In order to know the influence of CGO content in LSO, we attempt to calculate the density of composite by using the Archimedes principles. Table 2 shows that the higher the CGO content is, the higher the density of the composite.
Table 2: Density of sintered composite of LSO-CGO.
|
9LSO-1CGO |
8LSO-2CGO |
7LSO-3CGO |
|
| Mass (g) | 0.2859 | 0.2907 | 0.2957 |
| Radius (cm) | 0.3788 | 0.3740 | 0.3695 |
| thickness (cm) | 0.1565 | 0.1580 | 0.1635 |
| Volume (cm3) | 0.0705 | 0.0694 | 0.0701 |
| Density (g cm-3) | 4.0557 | 4.1890 | 4.2187 |
| Surface area (cm2) | 0.4508 | 0.4396 | 0.4291 |
It was concluded that the CGO helped to increase the electrolyte performance. In addition, the higher the density of LSO-CGO composite, the higher were their conductivities. A similar result was also presented in our previous research for LSO-YSZ composite.7,10
Conductivity investigation
The Nyquist plot obtained at 600, 650, and 700 °C for 9LSO-1CGO composite is presented in Figure 3.
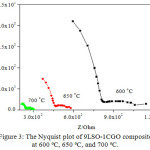 |
Figure 3: The Nyquist plot of 9LSO-1CGO composite at 600ºC, 650ºC, and 700ºC. |
The total resistance of composite rises about twice, when the operating temperature drops by 50 degrees. All impedance plots of an oxygen-ion conductor exhibit two semicircle parts. The third semicircle exhibit at Nyquist plot at 600 °C as a response to interface electrode-electrolyte at low-frequency region. The high-frequency region corresponds to the bulk response, whereas the medium-frequency region is related to the grain boundary contribution.8,11,12
Impedance measurement produces two impedances (R1 and R2). R1 is the contribution of its bulk phase response and R2 is the response of the grain boundary phase. For example, the total impedance 9LSO-1CGO composite at a temperature of 700°C measurement is 263.29 Ω. which is derived from the sum of a bulk phase response and phase between the grains. The bulk resistance region is larger than that of the grain boundary resistance. The temperature effect on the conductivity of LSO-CGO composite is shown at Figure 4.
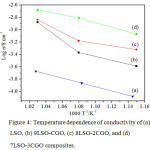 |
Figure 4: Temperature dependence of conductivity of (a) LSO, (b) 9LSO-CGO, (c) 8LSO-2CGO, and (d) 7LSO-3CGO composites. |
Figure 4 shows that composite conductivity is greatly dependent on both the temperature and the LSO:CGO ratio. The activation energies of 9LSO-1CGO, 8LSO-2CGO, and 7LSO-3CGO composites were 1.1 eV, 0.70 eV; and 0.60 eV, respectively. Overall, the activation energy below 1.1 eV suggests that the ionic conduction arises from the migration of interstitial oxide ions.5,13 The slope of the curve at high temperature states of energy required to create and move the intrinsic defects, whereas the slightly more curved slope shows the energy required to remove defects only.14 The conductivities and energy activation of the three series LSO-CGO composites are at 700°C are shown in Table 3. The composite of 7LSO-CGO shows a higher conductivity (2.10×10-3 at 700°C) and lower action energy compared to the other compositions.
Table 3: Conductivities of LSO-CGO composite at 700°C.
|
Composite |
R / Ω |
d / cm |
l / cm |
A / cm2 |
ρ/ S-1.cm |
σ / S cm-1 |
Ea / eV |
|
9LSO:1CGO |
263.29 |
0.7575 |
0.1565 |
0.4508 |
7578.01 |
1.32×10-3 |
1.1 |
|
8LSO:2CGO |
251.4 |
0.7480 |
0.1580 |
0.4396 |
6988.45 |
1.43×10-3 |
0.7 |
|
7LSO:3CGO |
181.4 |
0.7390 |
0.1635 |
0.4291 |
4756.40 |
2.10×10-3 |
0.6 |
This suggests that the higher the CGO content, the higher is the density of the composite. This is in consequence to the conductivities of series LSO-CGO composite.
Conclusion
A novel composite electrolyte, LSO-CGO was developed and used as the electrolyte for intermediate temperature fuel cells. The results showed that when the composition was 70 wt. % LSO and 30 wt. % CGO, the electrolyte had higher electrical conductivity in the temperature of 500-750oC. The activation energy values of synthesized composites were lower than 1.1 eV. This indicates that the composite material is possible for ion oxide conductor. This composite electrolyte is a promising electrolyte material for IT-SOFC’s.
Acknowledgment
The authors are grateful to Universitas Padjadjaran through Unpad internal grant (Hibah Internal Unpad) No 872/UNG.3.1/LT/2017.
References
- Badwal, S.P.S., Foger, K.: Solid Oxide Electrolyte Fuel Cell Review. Ceramics International 1996, 22, 257-265
CrossRef - Ormerod, R.M.: Solid oxide fuel cells. Journal of Chemistry Society Review 2002, 32, 17-28
CrossRef - Stambouli, A.B., Traversa, E.: Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy. Renewable and Sustainable Energy Reviews 2002, 6, 433–455
CrossRef - Sansom, J.E.H., Richings, D., Slater, P.R.: A powder neutron diffraction study of the oxide-ion-conducting apatite-type phases, La9.33Si6O26 and La8Sr2Si6O26. Solid State Ionics 2001, 139(3-4), 205-210
CrossRef - Noviyanti, A.R., Ismunandar, Prijamboedi, B., Marsih, I.N.: Hydrothermal Preparation of Apatite-Type Phases La9.33Si6O26 and La9M1Si6O26.5 (M = Ca, Sr, Ba). ITB Journal of Science 2012, 44((2)), 193-203
CrossRef - Liu, C., Zhang, H., Xia, J., Li, Z.: Synthesis and characterization of Zr0.85Y0.15O1.925-La9.33Si6O26 composite electrolyte for application in SOFCs. . Journal of Advanced Ceramics 2012, 1(4), 327-335
CrossRef - Noviyanti, A.R., Hastiawan, I., Irwansyah, F.S., Hidayat, S., Hardian, A., Syarif, D.G., Yuliyati, Y.B.: Preparation and Conductivity of Composite Apatite La9.33Si6O26(LSO) – Zr0.85Y0.15O1.925(YSZ) In: AIP Conference Proceedings Bandung Indonesia 2016, pp. 050002-050001-050002-050006. AIP Publishing
- Hardian, A., Choi, G.M., Suendo, V., Ismunandar: Structure and Ionic Conductivity of Codoped Ceria for IT-SOFC Electrolytes Journal of the Australian Ceramics Society Volume 2014, 50[2], 99 –109
- Yoshioka, H., Tanase, S.: Magnesium doped lanthanum silicate with apatite-type structure as an electrolyte for intermediate temperature. Solid State Ionics 176, 2395-2398, 2005.
CrossRef - Noviyanti, A.R., Hastiawan, I., Yuliyati, Y.B., Rahayu, I., Rosyani, D., Gustaman, S.D.: LSO apatite-YSZ composite as a solid electrolyte for solid oxide fuel cells. In: AIP Conference Proceedings 2017, pp. 040001-040001–040001-040006. AIP Publishing
- Bechade, E., Julien, I., Iwata, T., Massona, O., Thomasa, P., Championa, E.: Synthesis of lanthanum silicate oxyapatite materials as a solid oxide fuel cell electrolyte. Journal of European Ceramic Society 2008, 28, 2717-2724
CrossRef - Cao, X.G., Jiang, S.P.: Synthesis and characterization of lanthanum silicate oxyapatites co-doped with A (A ¼ Ba, Sr, and Ca) and Fe for solid oxide fuel cells. Journal of Materials Chemistry A 2014, 2, 20739–20747
- Islam, M.S., Tolchard, J.R., Slater, P.R.: An apatite for fast oxide ion conduction. Chemical Communations, 2003, 1486-1487
- West, A.R.: Solid State Chemistry and Its Applications, 2 ed. John Wiley & Sons, LTD, West Sussex PO19 8SQ, England, 1989.

This work is licensed under a Creative Commons Attribution 4.0 International License.









