Synthesis and Characterization of Benzimidazole by Using o-Phenylenediamine with Different Aldehydes and Carboxylic Acids in the Presence of ρ-TSOH as a Catalyst
Abdullah Jawad Kadhim and Ali Chazi Kazim
and Ali Chazi Kazim
Department of Chemistry, College of Education, University of Al-Qadisiyah, Iraq.
Corresponding Author E-mail: abdullah.kadhim@qu.edu.iq
DOI : http://dx.doi.org/10.13005/ojc/3404054
Article Received on : 23-03-2018
Article Accepted on : 27-05-2018
Article Published : 19 Jul 2018
This research paper deals with the synthesis and diagnose of Benzimidazole rings which were have been prepared by using two different methods in which used starting material o-phenylenediamine with different compounds. The first method is with aldehydes such as 4-Chlorobenzaldehyde, 4-N, N-Dimethylbenzaldehyde, and Formaldehyde. The second is with Carboxylic acids such as Salicylic acid, Acetic acid, and Butanoic acid. ρ-TSOH has been using as a catalyst in the synthesis methods above and used F.T.I.R and HNMR spectroscopy are used for diagnosing the prepared rings in addition to the physical properties.
KEYWORDS:Aldehydes; Benzimidazole; Carboxylic Acids; ρ-TSOH
Download this article as:| Copy the following to cite this article: Kadhim A. J, Kazim A. C. Synthesis and Characterization of Benzimidazole by Using o-Phenylenediamine with Different Aldehydes and Carboxylic Acids in the Presence of ρ-TSOH as a Catalyst. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Kadhim A. J, Kazim A. C. Synthesis and Characterization of Benzimidazole by Using o-Phenylenediamine with Different Aldehydes and Carboxylic Acids in the Presence of ρ-TSOH as a Catalyst. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47442 |
Introduction
Benzimidazole is one of the heterocyclic compounds that shows different biological qualities such as antibacterial and antifungal.1 Also, some Benzimidazoles have an effect on human viruses such as cytomegalovirus.2 There are two procedures for the synthesis of 2-substituted Benzimidazoles. The first is the reaction of phenylenediamines and carboxylic acids or its derivatives by heating3 in strong drying conditions.4 The second includes a two-step procedure that includes the oxidative cyclodehydrogenation of Schiff bases, which are often generated from the condensation of phenylenediamines and aldehydes3 and with aryl-aldehydes by using an acidic agent and also with silica gel at room temperature.4 p-TsOH has been used as a neutral acid catalyst to synthesize a number of benzimidazoles.5 It is considered as an important, effective, available and inexpensive incentive.6 Also, there are many ways to synthesize benzimidazole by using different catalysts such as Nanocrystalline oxides (MgO) with iodine,7 H2O2/HCl and Cu (OTf)2,8 HCl.9 In this work, the aim was the synthesis of benzimidazole rings by using one catalyst is p-TsOH as in previous studies above, which are used one catalyst in different circumstances.
Instruments
Melting points have been determined by using Melting point SMP3 apparatus.
F.T.I.R spectra have been recorded by using Fourier Transform Infrared Spectrophotometre (F.T.I.R) 8400 S Shimadzu apparatus.
HNMR spectra have been recorded by using NMR Spectrometer 400 MHz, Avance III 400 Bruker, Germany.
U.V. spectra havebeen recorded by using U.V-Visible Spectrophotometer 1650 PC Shimadzu apparatus.
Experimental Section
The General Method for Synthesizing Benzimidazoles from Aldehydes
A solution of Aldehyde (0.01 mole), o-phenylenediamine(0.01 mol) and DMF (3 ml) has been mixed with p-TsOH (20 ml). The mixture has been heated and stirred at 80°C for 2-3hr., then cooled to reach room temperature, the mixture has been added dropwise with stirring into mixture of Na2CO3 (0,01mole) and H2O (20 ml), the product has been filtered, washed by H2O, then dried2 to yield A, B and C compounds.
The General Method for Synthesizing Benzimidazole from Carboxylic Acids
A solution of Carboxylic acid (0.01 mole), o-phenylenediamine(0.01 mole) and toluene (10 ml) has been mixed with p-TsOH (20 ml). The mixture has been refluxed for 2-3 hr. then cooled, filtered and dried10 to yield D, E and F compounds.
Table 1: Physical Properties of Compounds
|
Compounds |
Molecular formula |
Solvent |
Yield % |
M. P. Cº |
Color |
|
A |
C13H9N2Cl |
DMF |
78 |
294-298 |
Brown |
|
B |
C15H15N3 |
DMF |
72 |
237-240 |
Nutty |
|
C |
C6H6N2 |
DMF |
85 |
115-118 |
Yellow |
|
D |
C13H10N2O |
Toluene |
81 |
151-157 |
Yellow |
|
E |
C8H8N2 |
Toluene |
72 |
Oil |
Nutty |
|
F |
C10H12N2 |
Toluene |
68 |
249 dec. |
Nutty |
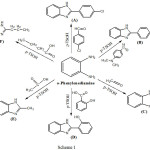 |
Scheme 1 |
Result and Discussion
Synthesis compounds characterized by F.T.I.R. and H-NMR spectroscopy have showed an important absorption packs of functional groups for the synthesis Benzimidazole derivatives from aldehydes and carboxylic acids with presence of p-TSOH as a catalyst in the different conditions.
2-(4-Chlorophenyl) -1H-Benzimidazole
The F.T.I.R spectrum of this compound shows appearance of an absorption pack at 3379cm-1 refers to (N-H) group and appearance of an absorption pack at 686 cm-1 refers to (C-Cl) bond. HNMR (400 MHz ) δ(ppm) Benzi. (7.26t 2H) and (7.71t 2H), Ar (7.31 and 7.61), N-H(3.42s). Table (2) shows the other absorption packs of the compound in the F.T.I.R spectrum.
2-(4-N,N- Dimethylaniline)-1H-Benzimidazole
The F.T.I.R spectrum of this compound shows an absorption pack at 3301cm-1 refers to (N-H) group and appearance of an absorption pack at 2916 cm-1 refers to (C-H) aliphatic. H-NMR (400 MHz) δ(ppm) Ar (6.91 and 7.31), Benzi. (7,26t 2H 7.69t 2H), N-H(4.42d), CH3(2.91). Table(2) shows the other absorption packs of the compound in the F.T.I.R spectrum.
1H-Benzimidazole
The F.T.I.R spectrum of this compound shows an absorption pack at 3409 cm-1 refers to (N-H) group and an absorption pack at 2923 cm-1 refers to (C-H) aliphatic. HNMR (400 MHz ) δ(ppm) Benzi. (7.68t 2H) and (7.26-7.30t 2H), N-H(2.9Hz), Benz.-CH(7.90Hz). Table (2) shows the other absorption packs of the compound in the F.T.I.R spectrum.
2-(2-hydroxy phenyl)-1H-Benzimidazole
F.T.I.R spectrum of this compound shows an absorption pack at 3425 cm-1 refers to (O-H) group and appearance of an absorption pack at 1157 cm-1 refers to (C-O) bond in addition to an absorption pack (N-H) group at 3240 cm-1. HNMR (400 MHz ) δ(ppm) Benz. (7.26t 2H) and (7.75t 2H), Ar (7.35- 7.00), N-H(2,93s), O-H(7.97). Table (2) shows the other absorption packs of compound in F.T.I.R spectrum.
2-Methyl-1H-Benzimidazole
The F.T.I.R spectrum of this compound shows an absorption pack at 3178 cm-1 refers to (N-H) group and an absorption pack at 2916 cm-1 refers to (C-H) aliphatic. HNMR (400 MHz ) δ(ppm) Benz. (7.26t 2H and 7.71t 2H), N-H(2.45), CH3 (2.20s). Table (2) shows the other absorption packs of the compound in the F.T.I.R spectrum.
2-Propyl-1H-Benzimidazole
The F.T.I.R spectrum of this compound shows an absorption pack at 3209 cm-1 refers to (N-H) group and an absorption pack at 2962 and 2931 cm-1 to (C-H) aliphatic. HNMR (400 MHz ) δ(ppm) Benz. (7.29t 2H) and (7.71t 2H), N-H(4.28s), -CH2-CH2(2.42S), CH2-CH2-(1,43S), CH3(0.92S). Table (2) shows the other absorption packs of the compound in the F.T.I.R spectrum.
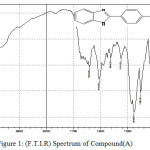 |
Figure 1: (F.T.I.R) Spectrum of Compound (A) |
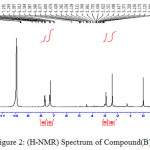 |
Figure 2: (H-NMR) Spectrum of Compound (B) |
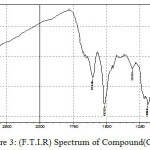 |
Figure 3: (F.T.I.R) Spectrum of Compound (C) |
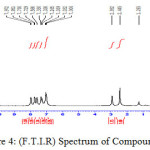 |
Figure 4: (F.T.I.R) Spectrum of Compound (D) |
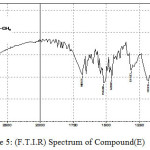 |
Figure 5: (F.T.I.R) Spectrum of Compound (E) |
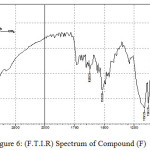 |
Figure 6: (F.T.I.R) Spectrum of Compound (F) |
Table 2: Other Absorption Pecks of Compounds in F.T.I.R. Spectroscopy.
|
Compounds |
Name of compound |
UV (nm) |
(C-H) aromatic cm-1 |
(C=N)(C=C) |
(C-N) |
|
A |
2-(4-Chlorophenyl) -1H-Benzimidazole |
318-379 |
3062 |
1512-1604 |
1311 |
|
B |
2-(4-N,N– Dimethylaniline)-1H-Benzimidazole |
293-374 |
3039 |
1496-1604 |
1365 |
|
C |
1H-Benzimidazle |
316 |
3062 |
1512-1604 |
1303 |
|
D |
2-(2-Hydroxy Phenyl)-1H-Benzimidazole |
293-396 |
3024 |
1666-1612 |
1249 |
|
E |
2-Methyl-1H-Benzimidazole |
357 |
3062 |
1519-1681 |
1311 |
|
F |
2-Propyl-1H-Benzimidazole |
357 |
3070 |
1512-1620 |
1157 |
Conclusion
In this study, a number of rings of benzimidazole have been synthesized from the reaction of o-phenylenediamine with different aldehydes and different carboxylic acids by using p-TSOH as a catalyst in all synthesis processes. The synthesis results have given a high percentage of the products with different solvent used. The spectral techniques FT.I.R and H-NMR have been used to diagnose the prepared rings.
Acknowledgments
The researcher gratefully acknowledges Chemistry department, University of Al-Qadisiyah, Iraq for providing lab facilities to carry out this work.
Reference
- Mohanraj, V.; Murugesan,V.; Karthik, A.; Aravindan, B. J. Env. Nanote., 2014 3, 1, 48-52.
- Xiangming, Han; Huiqiang, Ma; Yulu, Wang. ARKIVOC, 2007, (xiii), 150-154.
- Quiroga, Jairo; Nogueras, Manuel; Cobo, Justo. Europ. J. of Medi. Chem., 2011, 46, 4062-4070.
CrossRef - Chaturvedi, Amit K.; Negi, Arvind S.; Khare, Puja. RSC Advances, 2013, 3, 4500-4504.
CrossRef - Pasha, Mohamed Afzal; Nizam, Aatika. J. of Saud. Chem. Soc., 2011, 15, 55-58.
CrossRef - Mungra, Divyesh C.; Patel, Manish P.; Patel, Ranjan G.. Med Chem Res 2011 20, 782–789.
CrossRef - Naeimi, Hossein; Alishahi, Nasrin. J. of Experi. Nanosc., 2013, DOI:10.1080/17458080.2013.822575.
CrossRef - Tarpada, Umesh P.; Thummar, Bhautik B.; Dipak, K. Raval. J. of Saud. Chem. Soci., 2016 20, 530-535.
- Kadhim, Abdullah Jawad. Orient. J. Of Chem., 2018, 34, 1, 473-481.
CrossRef - Dawood, Kamal M.; Abdel-Wahab, Bakr F. ARKIVOC. 2010 (i) 333-3.

This work is licensed under a Creative Commons Attribution 4.0 International License.









