Solid phase extraction of trace Cu(II)using Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) to Determination by FAAS
Ali Moghimi1*, Elmira Peykani2, Sussan Samadi2
1Department of Chemistry, Varamin( Pishva )Branch Islamic Azad University, Varamin, Iran 2Department of Chemistry, Shahre Rey Branch Islamic Azad University, Shahre Rey, Iran
DOI : http://dx.doi.org/10.13005/ojc/300336
Article Received on :
Article Accepted on :
Article Published : 16 Sep 2014
A simple, reliable and rapid method for preconcentration and modified solid phase C18 with Single-Walled Carbon Nano tubes with Naphtol-2-(Pyridylazo-2)-1 (PAN) in order to prepare an effective sorbent for the preconcentration and determination of copper. The sorption capacity of Naphtol-2-(Pyridylazo-2)-1 (PAN) -modified Single walled carbon nano tubes (SWCNTs) was 50 mg.g -1 and the optimum pH for the quantitative recovery of copper was found as 3.0. The optimum flow rate, sorbent amount and sample volume were1.0 mL.min-1, 300 mg and 50 mL, respectively. 5 mL of 4 mol.L-1 HNO3 was the most suitable eluent. The recommended method is simple and reliable for the determination of copper without any notable matrix effect and successfully applied to environmental water samples. The results of this study show that little amount of Cu2+ ion with the aid of minimum chemical substacnces, in a relatively short amount of time, and with high percentage of recovery, can be isolated and identified. In this method, the detection limits equal to 0.1µg.mL-1, were achieved with the experiment’s relative standard deviation of (n=10) equal to 1.25% factor prior to condensation equating to 125 and the column capacity of 205 µg for each adsorbent.The accuracy of the proposed method was compared with other standard methods, it became clear superiority. Similar experiments on natural samples under optimal conditions have proven the efficiency of this method at an industrial scale.
KEYWORDS:Copper(II); Preconcentration; Solid phase extraction; Single walled carbon nanotubes (SWCNTs). Flame atomic adsorption spectrometry (FAAS).
Download this article as:| Copy the following to cite this article: Moghimi A, Peykani E, Samadi S. Solid phase extraction of trace Cu(II)using Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) to Determination by FAAS. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Moghimi A, Peykani E, Samadi S. Solid phase extraction of trace Cu(II)using Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) to Determination by FAAS. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4676 |
Introduction
The determination of metal ions in natural samples such as waters, soils and biological fluids is very important part of environmental and public health studies. However, the direct determination of metal ions at trace level is limited due to their low level of concentration and matrix interferences. Flame atomic absorption spectrometry (FAAS) which has been continuously used for the determination of trace metal ions suffers from insufficient sensitivity for direct determination of metal ions in environmental samples. Therefore a preconcentration or separation step is frequently necessary to improve the detection limit and sensitivity. For this purpose several separation and preconcentration procedures have been developed for trace metal ion determination involving different analytical strategies. These methods include ion exchange 1liquid extraction 2, anodic stripping voltammetry 3, cloud point 4, coprecipitation 5 and solid-phase extractions 6–10,24. Among the various methods, solid-phase extraction has received more acceptances due to its simplicity, rapidity and attainability of large preconcentration factor.
Avariety of solid materials such as modified ion exchange resins 11–13, functionalized resins with chelating reagents14, activated carbon 15, zeolites 16, cellulose 17 and immobilized microorganisms on sepiolite 18 have been used for preconcentration of trace metals. Microcrystalline naphthalene 19–21 and benzophenone 22 have also been used as solid-phase for adsorptive extraction of metal ion complexes.
In this work a simultaneous solid-phase preconcentration method for the determination of copper, iron and copper by atomic absorption is described.Although, disadvantages such as significant chemical additives, solvent losses, complex equipment, large secondary wastes, unsatisfactory enrichment factors and high time consumption, limit the application of these techniques. These problems could be addressed by the development of modular and compact processes that provide adequate separation and preconcentration without complex processes. The solvent microextraction technique effectively overcomes these difficulties by reducing the amount of organic solvent as well as allowing sample extraction and preconcentration to be done in a single step. The technique is faster and simpler than conventional methods. It is also inexpensive, sensitive and effective for the removal of interfering matrices. Solvent microextraction is a form of solvent extraction with phase ratio values higher than 100. Compared with the conventional solvent extraction, microextraction may provide poorer analyte recovery, instead the concentration in the organic phase greatly enhances. In addition, the amount of the used organic solvent is highly reduced and only one step of manipulation is necessary, therefore, problems of contamination and loss of analytes vanishes.
Cloud point extraction (CPE)26–31, homogeneous liquid–liquid extraction (HLLE)32,33 and single drop microextraction (SDME)34–38 are fairly new methods of sample preparation which are used in separation and preconcentration of metals and can solve some of the problems encountered with the conventional pretreatment techniques.
In the previous researches, we demonstrated a novel microextraction technique, named dispersive liquid–liquid microextraction (DLLME), which was successfully used, for the extraction and determination of polycyclic aromatic hydrocarbons (PAHs), organphosphorus pesticides (OPPs) and chlorobenzenes in water samples39–41. DLLME is a modi- fied solvent extraction method and its acceptor-to-donor phase ratio is greatly reduced comparing with the other methods. In DLLME, the appropriate mixture of the extraction and disperser solvents is rapidly injected by syringe into aqueous samples containing analytes. Thereby, cloudy solution forms. In fact, the cloudy state results from the formation of fine droplets of the extraction solvent, which disperse in the sample solution. Then, this cloudy solution shall be centrifuged and the fine droplets sediment at the bottom of the conical test tube. The determination of anlaytes in sedimented phase can be performed by instrumental analysis. In this extraction method any component in the solution, directly or indirectly after previous (or simultaneous) derivatization reaction, interacts with the fine droplets of the extraction solvent and consequently gets extracted from the initial solution and concentrates in the small volume of the sedimented phase. Simplicity of the operation, rapidity, low sample volume, low cost, high recovery and high enrichment factor are some advantages of DLLME.
DLLME is a miniaturized sample pre-treatment technique. On the other hand, graphite furnace atomic absorption spectrometry (GF AAS) is a micro amount sample analysis technique. Therefore, it makes it perfect when a combination of both DLLME and GF AAS is used. The applicability of the approach has been demonstrated for the determination of copper in water samples. This element was selected for evaluation of the procedure because copper is one of the principal heavy metals of analytical interest due to its extreme toxicity even at relatively low concentrations42,43.
In our knowledge, SPE and preconcentration by Cu(II)-imprinted diazoaminobenzene–vinylpyridine copolymer packed-bed columns have not been employed for the separation and preconcentration of Cu(II) from aqueous solution. This paper reports the synthesis of Cu(II) imprinted and non-imprinted copolymers by copolymerizing copper chloride (or without it), diazoaminobenzene (DAAB) and vinylpyridine (VP) using ethyleneglycol dimethacrylate (EGDMA) as cross-linker in presence of 2,2′- azobisisobutryonitrile as initiator and its analytical applications for column preconcentrative separation of Cu(II) from natural water.
This work preconcentration of Cu2+ based on the adsorption of its Naphtol-2-(Pyridylazo-2)-1 (PAN) complex on an Single walled carbon nanotubes (SWCNTs). The adsorbed complex could be eluted using environmentally and the concentration of Cu2+ was determined by FAAS. The influence of various experimental parameters such as acidity, sample volume, flow rate, diverse ions, etc. was examined in detail. The validity of the proposed method was tested in tap water samples and waste water.
Experimental
Instrumentation
Determination of Cu2+ contents in working samples were carried out by a PG-990 flame atomic absorption spectrometer equipped with a high intensity hallow cathode lamp(HI-HCl) according to the recommendations of the manufacturers. These characteristics are tabulated in( Table 3). The pH measurements were carried out by an pH meter (sartorius model PB-11).
Chemicals and reagents
Single walled carbon nanotubes (SWCNTs) and Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) were prepared from Merck (Darmstadt, Germany). Method and dried for a week over phosphorus pentoxide in a vacuum desiccators before use. 4-Isocyanatobenzenesulfonyl azide was prepared from 4-carboxybenzenesulfonyl azide via a published procedure [17]. All solutions were prepared with doubly distilled deionized water from Merck (Darmstadt, Germany). C18 powder for chromatography with diameter of about 50 µm obtained from Katayama Chemicals from supelco. It was conditioned before use by suspending in 4 M nitric acid for 20 min, and then washed two times with water.
 |
Schematic1: Synthesis and Molecular structure of Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) Click here to View Scheme |
Preparation of Single walled carbon nanotubes (SWCNTs) cartridge
A glass column 1.5 cm in diameter and 15 cm in length was used for the preconcentration of Copper(II). About 5 g of Single walled carbon nano tubes (SWCNTs) cartridge was mixed with 25mL of HCl 1M to form slurry and then loaded on to the column. Cotton was placed at the bottom for allowing Single walled carbon nanotubes (SWCNTs) cartridge to settle properly. The column was packed up to a height of 3 cm.
Procedure for preconcentration
A 50mg SDS and 30 mg Naphtol-2-(Pyridylazo-2)-1 (PAN) of 50mL was loaded on to the column of neutral Single walled carbon nanotubes (SWCNTs) cartridge maintaining a flow rate of 5mL min−1. Then250 ml volume of 10 µgm Cu(II) solution was loaded on to the column. The sample solution was loaded on to the column of neutral Single walled carbon nanotubes (SWCNTs) cartridge maintaining a flow rate of 0.5mL min−1. The complex was adsorbed as a narrow band on the top of the column. The adsorbed complex was eluted using 10mL of HNO3 4M at a flow rate of 0.5mL min−1 and the concentration of Copper(II) was determined by FAAS.
Results and discussion
The treatment of Single walled carbon nanotubes (SWCNTs) can lead to the Naphtol-2-(Pyridylazo-2)-1 (PAN) functional groups via adsorption [20] (Fig.1) .The formation of SWCNTs was followed by Raman Spectroscopy spectroscopy. Initially, in the spectrum of GO, the carbonyl vibration appears at 1580 cm-1, while there are fingerprints at 160 cm-1 and 230 cm-1 due to the presence of hydroxyl species at the carbon nanotubes. [23] (Fig.2).
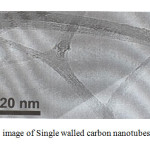 |
Fig1: SEM image of Single walled carbon nanotubes (SWCNTs) Click here to View Figure |
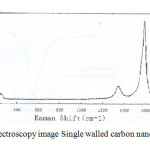 |
Fig2: Raman Spectroscopy image Single walled carbon nanotubes (SWCNTs) Click here to View Figure |
Effect of pH in does not occur.
The effect of pH of the aqueous solution on the extraction of 100 ng of each of the cations Cu(II) was studied in the pH rang of 1-10. The pH of the solution was adjusted by means of either 0.01 M H NO3 or 0.01M NaOH. The results indicate that complete chelation and recovery of Cu(II) occurs in pH range of 2-4 and that of in 2-8 and are shown in Fig. 3. It is probable that at higher pH values, the cations might be hydrolysed and complete desorption occur. Hence, in order to prevent hydrolysis of the cations and also keeping on the cartridge C18, pH=3.0 was chosen for further studies.
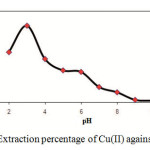 |
Fig3: Extraction percentage of Cu(II) against pH. |
Choice of the eluent
A variety of reagents were tested in order to elute the adsorbed complex from the column. In order to choose the most effective eluent for the quantitative recovery of Copper(II), methanol, ethanol, acetone, HCl 1M, HCl 3M ,H3PO4 1M, and Acidified methanol(Acidified solvents obtained by addition of HNO3 4M ), were studied. The adsorption studies were carried out maintaining an overall Cu(II) concentration of 10µg in 100mL sample volume. The recovery of Copper(II) was found to be quantitative with ethanol and HNO3 4M as eluting agents. However, HNO3 4M was preferred owing to its non-inflammability and less toxicity16,17. It was observed that when the ratio of HNO3 4M a recovery of 99.7% could be attained.
Effect of sample volume
The effect of sample volume on the recovery of the analyte was investigated in the range 100–1500mLmaintaining an overall concentration of 0.025 mol L−1 sulfuric acid. The resulting complex was eluted using 10mL of HNO3 4M. The results are presented in. As can be seen from the figure, it is evident that the recovery of Copper(II) is quantitative (>97%) up to 250mL sample volume. A preconcentration factor of 125 could be attained for quantitative recovery (>97%) of Cu(II) when the sample volume was 250 mL.
Effect of flow rate
The flow rate of 1–7 mL min−1 was found to be suitable for optimum loading of Cu(II) Naphtol-2-(Pyridylazo-2)-1 (PAN) complex on the Single walled carbon nanotubes (SWCNTs) cartridge. At higher flow rates, there was a reduction in the percentage adsorption of Copper(II). This could be probably due to the insufficient contact time between the sample solution and Single walled carbon nanotubes (SWCNTs) cartridge. A flow rate of 5mL min−1 was maintained for the elution of Copper(II) Fig 4.
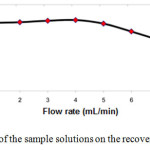 |
Fig4: Effect of flow rates of the sample solutions on the recovery percentage of Copper(II). Click here to View Figure |
Effect of the amount of Single walled carbon nanotubes (SWCNTs) silica cartridge
The amount of Single walled carbon nanotubes (SWCNTs) cartridge loaded was varied from 0.25 to 2.0 g and the preconcentration studies were carried as before. Quantitative recovery of Cu(II) could be attained in the range 0.75–2.0 g of Single walled carbon nanotubes (SWCNTs) cartridge. For amounts less than 0.75 g there was a significant reduction in the recovery beyond a sample volume of 100 mL.
Precision studies and limit of detection
The precision studies were carried out at 10 µg level of Copper(II) by carrying out 10 separate determinations using the above-mentioned procedure. The sample volume was maintained at 100 mL. The relative standard deviation of the method was found to be 1.25%. The sensitivity of the developed method is reflected by the limit of detection studies, defined as the lowest concentration of Cu(II) below which quantitative recovery of the metal ion by Single walled carbon nanotubes (SWCNTs) cartridge is not perceptibly seen. The limit of detection was found to be 0.1µg.mL-1.
Stability of the column
The stability of the column was tested using 10µg Cu(II) maintaining a sample volume of 50 mL. The adsorbed Cu(II) Naphtol-2-(Pyridylazo-2)-1 (PAN) complex on SDS was eluted using 6mL of HNO3 4M. The column could be used with good precision and quantitative recovery (>97%) for at least 10 cycles. Beyond 10 cycles, there was a significant reduction in the recovery of Copper(II).
Effect of other ions
The interfering effect of diverse ions was studied at varying concentrations. The preconcentration studies were carried out as mentioned above using 10µg Cu(II) maintaining a sample volume of 100 mL. The studies indicated that Na+, K+, Ca2+, Mg2+,Cd2+,Cl–, Br–, Fe3+, NO3−, Zn2+, Co2+, Ni2+,Mn2+did not cause any significant reduction in the recovery of Copper(II). The results are presented in Table 1 showing the recovery of Cu(II) with varying concentrations of metal ions. The recovery was found to be quantitative in the concentration range of the metal ions that was investigated. Since, the ions that are commonly present in water samples did not interfere significantly, the method was applied to study the recovery of Copper(II) in water samples.
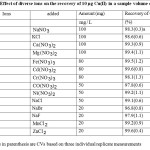 |
Table1: Effect of diverse ions on the recovery of 10 µg Cu(II) in a sample volume of 100 mL Click here to View table |
Recovery studies in tap water and rain water samples
The validity of the proposed method was tested by spiking known concentrations of Copper(II) to tap water(Tehran, taken after 10 min operation of the tap), and rain water (Tehran, 31 January, 2013) samples. The water samples were filtered and stored in polythene bottles. The recovery of Copper(II) was found to be satisfactory with a relative standard deviation of 2% for five replicate measurements and the results are shown in Table 2.
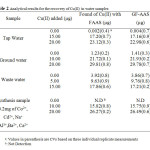 |
Table2: Analytical results for the recovery of Cu(II) in water samples Click here to View table |
Comparison with other solid phase adsorbents
The proposed methodology was compared to a variety of solid adsorbents reported recently in the literature. Also, the proposed method was free of interference compared to conventional procedures to determine Copper.46,51,52 As can be seen from the references, it is evident that the preconcentration factor obtained with Single walled carbon nanotubes (SWCNTs) cartridge is comparable to or even better than most of the other chelating matrices. The other significant feature of the proposed method is the use of environmentally benign HNO3 4 M for the elution of the complex.
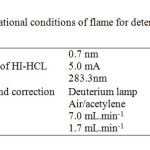 |
Table3: The operational conditions of flame for determination of Copper Click here to View table |
Calibration curve
The calibration curve of Copper(II) solutions is represented in Fig.5 and the related regression is Y=0.0147X+0.011 providing a correlation coefficient of R2=0.9998.
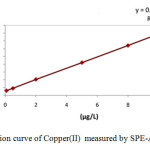 |
Fig5: Calibration curve of Copper(II) measured by SPE-AAS. Click here to View Figure |
Conclusions
The proposed spectrophotometric method for Copper(II) is simple, sensitive and exhibits good selectivity. The elution of the complex does not involve strong acids or toxic organic solvents. The advantage of using HNO3 4 M as the eluent lies in the fact that it is non-inflammable, inexpensive and non-toxic. The conventional solvent extraction procedure associated with metal dithizonates is avoided in this methodology. The highest preconcentration factor attainable was 125 for a 250mLsample volume. The method showed minimum interferences with commonly found ions in water sample and the recovery of Copper(II) was quantitative. The important features of the proposed method are its higher adsorption capacity with good preconcentration factor. The developed method is sensitive in detecting Cu(II) at ppb levels. The column could be used with good precision and quantitative recovery for at least 10 cycles. The quantitative recovery of Copper(II) with a low relative standard deviation of 1.25%reflects the validity and accuracy of the proposed method when applied to real samples. The method developed was simple, reliable, and precise for determining Copper in water. Also, the proposed method was free of interference compared to conventional procedures to determine Copper.46,51,52
Acknowledgments
We gratefully acknowledge financial of department of chemistry, department of Shahr Rey branch Islamic Azad University for financial support.
References
- H. Filik, B.D. Ozturk, M. Dogutan, G. Gumus, R. Apak, Talanta 44 (1997) 877.
- Kokal, V. Synek, P. Jano, Talanta 58 (2002) 325.
- C. Locatelli, G. Torsi, Microchem. J. 78 (2004) 175.
- S.A. Kulichenko, V.O. Doroschuk, S.O. Lelyushok, Talanta 59 (2003) 767.
- S. Kagaya, Z.A. Araki, Y. Hasegawa, Anal. Sci. 18 (2002) 923.
- A. Goswami, A.K. Sigh, Talanta 58 (2002) 669.
- G. Yang, Zh. Huang, Q. Hu, J. Yin, Talanta 58 (2002) 511.
- K.S. Abou-El-Sherbini, I.M.M. Kenawy, M.A. Ahmed, R.M. Issa, R. Elmorsi, Talanta 58 (2002) 289.
- M.E. Mahmoud, M.S.M. Saadi, Anal. Chim. Acta 450 (2001) 239.
- S. Saracoglu, L. Elci, Anal. Chim. Acta 452 (2002) 77.
- M.C. Yebra, J. salgado, L. Ouig, A. Moreno-Cid, Anal. Bioanal. Chem. 374 (2002) 530.
- A. Uzan, M. Soylak, L. Elc¸i, Talanta 54 (2001) 197.
- S. Sarac¸olu, L. Elc¸i, Anal. Chim. Acta 452 (2002) 77.
- H.J. Chang, Y.U.H. Sung, D.Sh. Huang, Analyst 124 (1999) 1695.
- S. Cerutti, M.f. Silva, J.A. Gasquez, R.A. Olsina, L.D. Martinez, Spectrochim. Acta Part B 58 (2003) 43.
- Y.P.d. Pena, W. Lopez, J.L. Burguera, M. Burguera, M. Gallignani, R. Brunetto, P. Carrero, C. Rondon, F. Imbert, Anal. Chim. Acta 403 (2000) 249.
- K. Zih-Perenyi, A. Lasztity, Zs. Horvath, A. Levai, Talanta 47 (1998) 673.
- H. Bag, A. Rehber T¨urker, M. Lale, Talanta 51 (2000) 1035.
- D. Thorburn Burns, N. Tungkananuruk, Anal. Chim. Acta 189 (1986) 383.
- B.K. Puri, S. Balani, Talanta 42 (1995) 337.
- Harvey Diehl, Clifford C. Hach. Inorg.Synth. Inorganic Syntheses 3: 196–201,(1950).
- Kaiss .R.,Waleed .F and Mohammed A., J.of Al-Anbar university for pure science : Vol.1 : No.1 ( 2007).
- A. Vogel, A Textbook of Quantitative Inorganic Analysis, 3rd ed., Longman, London, 1975.
- MOGHIMI, A.,” Preconcentration and Determination of Fe(III) Using Octadecyl SilicaMembrane Disks and Flame Atomic Absorption Spectrometry” Material Science Research India impress (2006).
- Nayebi, P.; MOGHIMI, A. Oriental Journal of Chemistry 2006,22(3),507.
- Hinze, W.L.; Pramaur, E.; Rev. Crit. ; Anal. Chem. 1993, 24 ,133.
- MOGHIMI, A. Chinese Journal of Chemistry 2007,25, 640.
- MOGHIMI, A., Chinese Journal of Chemistry 2007,25,10, 1536.
- MOGHIMI, A.; Shahryar Ghammamy “Environmental chemistry an Indian journal”2007,Vol.2, Issues 3
- MOGHIMI, A. Oriental Journal of Chemistry 2006,22(3),527.
- Zhu, X. ; Zhu, X. ; Wang, B. ; Microchim. Acta 2006,154, 95.
- Ghiasvand, A.R. ; Shadabi, S. ; Mohagheghzadeh, E. ; Hashemi, P. ; Talanta 2005,66,912.
- Igarashi, S.; Ide, N.; Takagai, Y.; Anal. Chim. Acta 2000,424 , 263.
- Fan, Z.; Zhou, W.; Spectrochim. Acta Part B 2006, 61 ,870.
- Li, L.; Hu, B.; Xia, L. ; Jiang, Z. ; Talanta 2006,70 ,468.
- Chamsaz, M.; Arabab-Zavar, M.H.; Nazari, S.; J. Anal. At. Spectrom. 2003,18 , 1279.
- Fragueiro, S.; Lavilla, I.; Bendicho, C.; Spectrochim. Acta Part B 2004, 59, 851.
- Fragueiro, S.; Lavilla, I.; Bendicho, C.; Talanta 2006,68 ,1096.
- Rezaee, M.; Assadi Y.; Milani Hosseini, M.R. ; Aghaee, E.; Ahmadi, F. ; Berijani, S.; J. Chromatogr. A 2005,1116 ,1.
- Berijani, S.; Assadi, Y. ; Anbia, M.; Milani Hosseini, M.R. ; Aghaee, E.; J. Chromatogr. A 2006,1123 ,1.
- Rahnama Kozani, R.; Assadi, Y.; Shemirani, F.; Milani Hosseini, M.R. ; Jamali, M.R.; Talanta, in press.
- P.Nayebi,; A.MOGHIMI, Oriental Journal of Chemistry 2006,22(3),507.
- J.L. Manzoori, M.H. Sorouradin, A.M.H. Shabani,Microchem. J. 63 (1999) 295–301.
- M.A. Taher, S. Puri, R.K. Bansal, B.K. Puri, Talanta45 (1997) 411–416.
- F. Shemirani, S.D. Abkenar, J. Anal. Chem. 59 (4) (2004) 327–330.
- M.A. Taher, Turk. J. Chem. 27 (2003) 529–537.
- J.L. Manzoori, G. Karim-Nezhad, Anal. Sci. 19 (4) (2003)579–583.
- P. Bermejo-Barrera, M.A. Nancy, D.L. Cristina, B.B. Adela, Microchim.Acta 142 (1–2) (2003) 101–108.
- Ali Moghimi; Oriental Journal of Chemistry 2006,22(3),527.
- Ali Moghimi.; Ghammamy S. “Environmental chemistry an Indian journal”2007,Vol.2, Issues 3.
- Choi,Y.S.;Choi,H.S.Bull.Korean Chem. Soc.24(2003)222.
- Saber Tehrani,M.; Rastegar,F.; Parchehbaf, A.;Rezvani,Z.;Chinese Journal of Chemistry 23(2005)1437.

This work is licensed under a Creative Commons Attribution 4.0 International License.









