Conductimetric Properties of Benzoic Acid in Water and 1-Propanol Mixtures at the Temperatures from 298.15 to 313.15K
Humayun Kabir1, Ranjit K. Nath2 , Kamrul Hossain3 and M. K. Mohammad Ziaul Hyder2
, Kamrul Hossain3 and M. K. Mohammad Ziaul Hyder2
1Department of Chemistry, Comilla Cadet College, Comilla-3503, Bangladesh.
2Department of Chemistry, Faculty of Engineering and Technology, Chittagong University of Engineering and Technology, Chittagong-4349, Bangladesh.
3Department of Chemistry, Faculty of Science, University of Chittagong, Chittagong-4331, Bangladesh.
Corresponding Autthor E-mail: rkn_chem@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340122
Molar Conductance of benzoic acid 1-propanol-water mixtures has been measured in the composition of 0, 5, 10, 15, 20, 30 and 40 wt% of 1-prpanol at 5K interval from 298.15 to 313.15K. The limiting molar conductance Λo and thermodynamic dissociation constantpKa was estimated from the measured conductance data using Bray-Kraus and Fuoss-Kraus equations. An approximate linear dependence of pKa with the reciprocal of the dielectric constant of the media was observed and this trend may be due to the dominating effect of the coulombic forces of attractions between the opposite ions over the other types of attractions. The gradual decreases of Λowith the increase of 1-propanol fraction in the binary mixtures of 1-propanol and water may be attributed to relative increase in the viscosities of 1-propanol-water mixtures. The Walden product and normalized Walden product (nwp) of the benzoic acid in the seven binary mixtures have been calculated and a qualitative proposal has been made regarding their variation with wt% of 1-propanol. The free energies of transfer ∆G°tr, also have been computed for the acid in the solvent mixtures. The change of pKa with the solvent composition has been discussed in terms of the free energy of transfer from water to the 1-propanol-water mixtures.
KEYWORDS:Binary Solvent; Benzoic Acid; Thermodynamic Dissociation Constant
Download this article as:| Copy the following to cite this article: Kabir H, Nath R. K, Hossain K, Hyder M. K. M. Z. Conductimetric Properties of Benzoic Acid in Water and 1-Propanol Mixtures at the Temperatures from 298.15 to 313.15K. Orient J Chem 2018;34(1). |
| Copy the following to cite this URL: Kabir H, Nath R. K, Hossain K, Hyder M. K. M. Z. Conductimetric Properties of Benzoic Acid in Water and 1-Propanol Mixtures at the Temperatures from 298.15 to 313.15K. Orient J Chem 2018;34(1). Available from: http://www.orientjchem.org/?p=43395 |
Introduction
The binary solvent systems are important for many practical applications, such as, in HPLC analysis,1 in MALDI-TOF analysis,2in the study of several physicochemical properties of electrolyte solutions.3It has attracted the researchers to study the effect of physical properties of solvents on dissociation of acids and electrolytes. Literarure reveals that few studies on the dissociation constants of benzoic acid and derivative of benzoic acids in alkanol-water mixtures by conductometric methods have been performed earlier.4-8But no report about the investigation on the conductometric studies of benzoic acid in 1-propanol mixture are available yet.
Conductometric studies can be used to determine the purity of solvent, relative ionic strength, dissociation constant of acids, solubility product etc. 9The present paper reports molar conductance of dilute solution of benzoic acid in 1-propanol-water binary mixtures ranging in composition from 0 to 40 wt% 1-propanolin waterat 5K interval from 298.15 to 313.15K. Thermodynamic dissociation constant pKa was estimated from the measured conductance data using Bray-Kraus10 and Fuoss-Kraus11 equations. From the relation between the pKa and the composition of the solvent mixtures, solute-solvent interaction can be assessed. The free energy of transfer ∆Gotr of carboxylate ions from water to alcohol-water mixtures is also discussed.
Materials and Method
Chemicals
Benzoic acid (BDH), 99.9% pure was used without further purification. 1-Propanol (Fisher chemicals, Fisher Scientific UK. Ltd.) was used for the preparation of mixed solvents without further distillation. The specific conductance of 1-propanol was measured to be 0.3-0.4×10-6ohm-1cm-1at room temperature. Doubly distilled water was used for preparing the mixed solvents. The specific conductance of water was measured to be 1.2-1.5 ×10-6 ohm-1 cm-1 at room temperature.
Preparation of Mixed Solvents and Benzoic Acid Solutions
1-propanol-water mixed solvents were prepared in weight-by-weight ratio, using measured volumes of the liquids multiplied by their respective densities at the temperature of mixing (room temperature). Density values of the liquids were used from the standard charts.
A stock solution of the acid was prepared by dissolving weighed amount of the solute in a definite volume of the solvent, secondary stock solutions were prepared from this solution by dilution. During conductance measurement, different aliquot of the stock acid solutions were added to a suitable volume of the solvent taken in a glass cell to prepared solutions of different concentrations.
Apparatus
Thermostatic water bath having a temperature accuracy of + 0.050C was used for equilibrating all solutions before conductance measurement. A simple glass cell of approximately 50 ml capacity with an adjustable plastic covering was used for taking the solutions for conductance measurement. Conductometer, model DDS-307 with dip type cell was used for conductance measurement. The accuracy of the cell was ± 1%.The Specific conductance of a solution was directly read out from the meter.
Procedure
A suitable volume of the solvent was pipetted into pyrex glass cell, placed in the thermostatic bath and properly clamped. A small aliquote of the stock acid solution pipetted into the glass cell with help of a 1 mL pipette. The mixture was mixed properly with the cell electrode and equilibrated to the bath temperature. Specific conductance of the solution was read directly from the conductivity meter. In this way, solutions of gradually decreasing concentrations were prepared by adding more of the 1-propanol-water solvent mixtures and their specific conductance was measured.
Result and Discussion
The dissociation constant (Ka) of benzoic acid was investigated at seven different percent of 1-propanol in the water-propanol mixtures in the range of 0-40% of 1-propanol from 298.15 to 313.15 K at 5 K interval. The molar conductances (Λ) of benzoic acid at different concentrations (C) of benzoic acid are given in Table1.
The experimental molar conductances of benzoic acid were first analyzed by Bray-Kraus10 Equation1 to obtain the limiting molar conductance (Λ).

Λ° valuesfor each percent 1-propanol solution at each temperature calculated from the intercept of the plots of (1/Λ) vs.(Λ x C) values from the Bray-Kraus equation were then used to calculate Ka values of benzoic acid from the least square fitting of the Fuoss-Kraus11 Equation 2.

Where, Fuoss function F(z) and activity coefficient f± calculated as the stated by Fuoss-Kraus model.11 Thus, from the plot of
![]()
versus
![]()
the slope
![]()
can be obtained. From the values of slope Ka was calculated.
Table 1: Molar conductance() of benzoic acids in 1-propanol-water mixtures at different temperatures.
| Concentration(Mole L-1) x104 | Molar conductance ()/ ohm-1cm-1 | |||
| T/K | T/K | T/K | T/K | |
| 298.15 | 303.15 | 308.15 | 313.15 | |
| 0 wt% of 1-propanol | ||||
| 3.830 | 112.80 | 110.97 | 108.88 | 114.63 |
| 3.556 | 115.85 | 114.44 | 112.20 | 118.10 |
| 3.319 | 119.00 | 117.50 | 115.39 | 121.41 |
| 3.112 | 122.12 | 120.83 | 118.58 | 124.37 |
| 2.929 | 125.31 | 123.60 | 121.21 | 127.36 |
| 2.766 | 127.98 | 126.54 | 124.01 | 130.15 |
| 2.620 | 130.90 | 128.99 | 126.70 | 132.80 |
| 2.489 | 132.96 | 131.36 | 129.35 | 135.37 |
| 5 wt% of 1-propanol | ||||
| 5.234 | 85.40 | 81.27 | 80.00 | 78.92 |
| 4.972 | 87.49 | 83.11 | 81.97 | 80.82 |
| 4.735 | 89.12 | 84.87 | 83.87 | 82.66 |
| 4.520 | 90.93 | 86.58 | 85.53 | 84.47 |
| 4.144 | 94.12 | 88.27 | 87.39 | 86.06 |
| 3.825 | 97.26 | 91.47 | 90.74 | 89.30 |
| 3.552 | 100.24 | 94.91 | 93.60 | 92.55 |
| 3.315 | 103.17 | 97.70 | 96.29 | 95.17 |
| 10 wt% of 1-propanol | ||||
| 6.577 | 58.38 | 58.23 | 57.02 | 55.80 |
| 6.166 | 60.17 | 59.84 | 58.71 | 57.41 |
| 5.803 | 61.69 | 61.52 | 60.31 | 59.10 |
| 5.481 | 63.13 | 62.94 | 61.85 | 60.57 |
| 5.193 | 64.71 | 64.52 | 63.36 | 62.01 |
| 4.933 | 66.09 | 65.88 | 64.67 | 63.25 |
| 4.485 | 68.90 | 68.68 | 67.57 | 66.01 |
| 4.111 | 71.28 | 71.28 | 69.82 | 68.60 |
| 15 wt% of 1-propanol | ||||
| 7.129 | 42.643 | 41.381 | 41.661 | 42.082 |
| 6.654 | 44.036 | 42.684 | 42.984 | 43.435 |
| 6.238 | 45.369 | 43.926 | 44.246 | 44.727 |
| 5.871 | 46.672 | 45.139 | 45.650 | 45.990 |
| 5.545 | 47.793 | 46.531 | 46.711 | 47.072 |
| 5.253 | 48.923 | 47.594 | 47.974 | 48.355 |
| 4.990 | 50.098 | 48.695 | 48.896 | 49.497 |
| 4.537 | 52.243 | 50.700 | 51.141 | 51.582 |
| 20 wt% of 1-propanol | ||||
| 6.499 | 30.238 | 30.007 | 29.391 | 30.469 |
| 6.092 | 31.104 | 30.202 | 31.351 | 30.940 |
| 5.734 | 32.002 | 31.217 | 32.351 | 31.828 |
| 5.415 | 32.869 | 32.038 | 33.146 | 32.685 |
| 4.874 | 34.469 | 33.649 | 34.777 | 34.264 |
| 4.431 | 35.998 | 35.095 | 36.337 | 35.772 |
| 4.062 | 37.547 | 36.562 | 37.793 | 37.301 |
| 3.749 | 38.808 | 37.601 | 37.875 | 39.075 |
| 30 wt% of 1-propanol | ||||
| 8.367 | 14.103 | 14.283 | 14.462 | 14.641 |
| 7.902 | 14.490 | 14.844 | 15.034 | 14.667 |
| 7.486 | 14.868 | 15.228 | 15.055 | 15.415 |
| 7.112 | 15.228 | 15.425 | 15.608 | 15.791 |
| 6.773 | 15.591 | 15.960 | 16.152 | 15.783 |
| 6.465 | 15.926 | 16.117 | 16.318 | 16.504 |
| 6.184 | 16.251 | 16.445 | 16.639 | 16.849 |
| 40 wt% of 1-propanol | ||||
| 8.687 | 8.611 | 8.806 | 8.944 | 9.082 |
| 8.325 | 8.781 | 9.129 | 9.273 | 8.985 |
| 7.992 | 8.946 | 9.309 | 9.459 | 9.159 |
| 7.685 | 9.122 | 9.486 | 9.642 | 9.330 |
| 7.400 | 9.297 | 9.662 | 9.811 | 9.500 |
| 7.136 | 9.459 | 9.824 | 9.978 | 9.683 |
| 6.890 | 9.623 | 9.841 | 10.000 | 10.027 |
The limiting molar conductance (Λo) value of benzoic acid in aqueous medium was found to be 362.98 S cm-1mol-1.Niazi et al12 reported the Λo for benzoic acid to be 383.55 ± 1.16 S cm-1mol-1. Strong et al13 have also reported Λo for benzoic acid to be 383.14 ±1.76 . Table 2 shows that with the increase of the proportion of 1-propanol in the 1-propanol-water mixtures the Λo values of benzoic acid decrease. This type of observed result may be due to the relative increase in the viscosities of 1-propanol-water mixtures. The decrease of Λo values may be the resultant of their overall effect facilitating the thermal movement of the ions.
Table 2: Λo Values for Acids in Alcohol-Water Mixtures at Different Temperatures.
| T/K | Λo / ohm-1cm-1 | ||||||
| 0 wt% | 5 wt% | 10 wt% | 15 wt% | 20 wt% | 30 wt% | 40 wt% | |
| 298.15 | 362.98 | 315.50 | 270.53 | 263.47 | 229.29 | 143.41 | 151.75 |
| 303.15 | 364.70 | 324.63 | 272.72 | 275.12 | 238.78 | 138.16 | 144.17 |
| 308.15 | 368.60 | 335.23 | 280.31 | 286.89 | 260.42 | 134.31 | 142.54 |
| 313.15 | 369.55 | 347.55 | 286.40 | 284.22 | 219.68 | 128.87 | 136.14 |
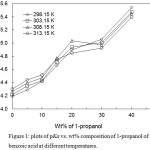 |
Figure 1: plots of pKa vs. wt% composition of 1-propanol of benzoic acid at different temperatures. Click here to View figure |
The pKa values calculated from the thermodynamic dissociation constant are presented in Table 3. The value tthermodynamic dissociation constant (pKa) of benzoic acid in aqueous solution was found 4.26 that were in complete agreement with the reported values.12,15The relation between the pKa values of the acid and the percent of 1-propanol in 1-propanol-water mixtures is shown in Figure 1.The pKa values increased with the increase in concentration of 1-propanolin the binary mixtures of 1-propanol-water. The increase in pKa values with the increase of the alcohol concentration (as dielectric constant decreases) may be due to dominating influence of coulombic forces of attraction over the dispersive force of attraction. Gloverset al.16 and Fong et al.17 determined the dissociation constant Ka of propionic acid in ethanol-water mixtures potentiometrically and observed a similar trend in the pKa vs. wt%. They also noticed a similar trend in the temperature variation of the pKa values similar to present investigation.
Table 3: pKaValues of Benzoic Acids in 1-propanol-Water Mixtures at Different Temperatures.
| T/K | pKaValues | ||||||
| 0 wt% | 5 wt% | 10 wt% | 15 wt% | 20 wt% | 30 wt% | 40 wt% | |
| 298.15 | 4.260 | 4.287 | 4.417 | 4.660 | 4.895 | 5.057 | 5.539 |
| 303.15 | 4.285 | 4.345 | 4.428 | 4.715 | 4.938 | 5.011 | 5.473 |
| 308.15 | 4.312 | 4.390 | 4.474 | 4.770 | 5.038 | 4.974 | 5.448 |
| 313.15 | 4.327 | 4.440 | 4.516 | 4.754 | 4.846 | 4.924 | 5.393 |
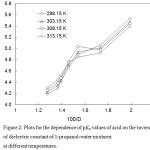 |
Figure 2: Plots for the dependence of pKa values of acid on the inverse of dielectric constant of 1-propanol-water mixtures at different temperatures. Click here to View figure |
The relation between the pKa values of benzoic acid and the inverse of dielectric constants of solvent mixtures is shown in Figure 2. From the figure it is shown that the plots are non linear. This nonlinearity indicates that there are some specific solute-solvent interaction among the ions and the solvent mixtures. This is further evident from the values of ∆Gotr for carboxylate ions on their transfer from water to co-solvent-water mixtures. The free energy of transfer of benzoic acid from water to 1-propanol-water mixtures are calculated from the equation, ∆Gotr = RT 2.303 (pKas– pKaw) where s and w referred to solvent mixtures and water respectively.The plots of the thermodynamic parameters for the transfer of benzoic acid from water to water-1-propanol mixtures are shown in Figure 3. These plots are also nonlinear. The values of the thermodynamic transfer energies are consistent if we examine the ∆Gotr with the dielectric constant of the media. With a lowering of the dielectric constant of the media by the addition of the 1-propanol in the 1-propanol-water mixtures, the force of attraction between the oppositely charged ions increases, thereby pKa increases, requiring higher energy to transfer the species from the aqueous to non-aqueous media. The plots of ∆Gotrvs. solvent mixtures have been found the same as reported by Niazi et al.12
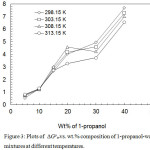 |
Figure 3: Plots of ∆Gotrvs. wt.% composition of 1-propanol-water mixtures at different temperatures. Click here to View figure |
The normalized Walden products (nwp) are calculated in our case at different wt% and at different temperatures. They are graphically presented in the Figure 4. A good agreement of the nwp was also observed by Niazi et al12 in case of benzoic acid and other acids in propanol- water and number of other aqueous–alcohol mixtures at 25oC. They concluded that this trend of the nwp values might not be due to the change of viscosities or dielectric constant, but also to an altering proton transfer mechanism as solvent is enriched with organic component. In our case there is no systemic trend in the nwp with the increase in 1-propanol concentration of the solvent mixtures. But nwp values of benzoic acid generally decrease with the increase in temperatures. Conway et al14observed that the nwp values passed through a maximum at about 15 to 25 wt% alcohol concentration in the solvent mixtures which is in good agreement with our results.
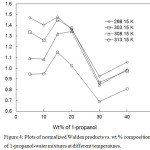 |
Figure 4: Plots of normalized Walden products vs. wt % composition of 1-propanol-water mixtures at different temperatures. Click here to View figure |
Acknowledgments
The Authors expresses honest appreciation to the Department of Chemistry, CU, Bangladesh for providing lab facilities for this research.
Conclusion
The solubility of benzoic acid in water increases with an increment of the propanol in water concentration which corresponds that the co-solvent of propanol enhanced the solubility of the benzoic acid. There is no regular trend of thermodynamics dissociation constant of benzoic acid in the range of temperature between 298.15 to 313.15K. Benzoic acid dissociation in propanol co-solvent is spontaneous above the temperature 298.15K.
References
- Hara, S., Fujii, Y., Hirasawa, M., Miyamoto, S. J. Chromatogr. A.1978, 149, 143-149.
CrossRef - Chen, H., Guo, B. Anal. Chem., 1997, 69, 4399–4404.
CrossRef - Kay, R.L., Broadwater, T.L., Electrochim. Acta. 1971, 16, 667-676.
CrossRef - Roses, M., Bonet, M.J., Bosch, E. Anal. Chim. Acta.1996, 333, 241-247.
CrossRef - Wang, H., Wang, Q., Xiong, Z., Chen, C., Shen, B. J. Chem. Thermodyn.2015, 83, 61-66.
CrossRef - Yurquina, A., Manzur, M. E., Brito, P., Manzo, R., Molina, M. A. A. J. Mol. Liq.2003, 108, 119-133.
CrossRef - Kumari, A., Sandeepa, K., Prathap, K. T., Satyavathi, B. J. Chem. Eng. Data. 2016, 61, 67-77.
CrossRef - Long, B., Li, J., Zhang, R., Wan, L. Fluid Phase Equilib.2010, 297, 113-120.
CrossRef - Glasstone, S. EWP, 1942.
- Kraus, C.A. and Bray, W.C.J. Amer. Chem. Soc.1913, 35, 1315.
CrossRef - Fuoss, R. M. and Kraus, C.A. J. Chem. Soc. 1933, 55, 476.
CrossRef - Niazi, M. S. K., Shah, S. S., Ali. J.and Khan. M. Z. I., J. Sol. Chem. 1990, 19, 623.
CrossRef - Strong, L. E., Blubaug, D., J. Kallmeyer J. B. and Taylor S. P. J. Phys. Chem.1985, 89, 1318.
- Conway, B.E., Bokris, J. O. M. and Linton, H. J. Phys. Chem.1956, 24, 834.
CrossRef - DASH, U. N. J. Indian Chem.Soc.1994, 71, 65-69.
- Glovers, D. J. J. Amer. Chem. Soc.1965, 87, 5275.
CrossRef - Fong, D. and Grunwald, E. J. Chem. Soc. 1969, 73, 3909.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

This work is licensed under a Creative Commons Attribution 4.0 International License.









